Abstract
Objective To investigate and quantify the potential dose-response association between egg consumption and risk of coronary heart disease and stroke.
Design Dose-response meta-analysis of prospective cohort studies.
Data sources PubMed and Embase prior to June 2012 and references of relevant original papers and review articles.
Eligibility criteria for selecting studies Prospective cohort studies with relative risks and 95% confidence intervals of coronary heart disease or stroke for three or more categories of egg consumption.
Results Eight articles with 17 reports (nine for coronary heart disease, eight for stroke) were eligible for inclusion in the meta-analysis (3 081 269 person years and 5847 incident cases for coronary heart disease, and 4 148 095 person years and 7579 incident cases for stroke). No evidence of a curve linear association was seen between egg consumption and risk of coronary heart disease or stroke (P=0.67 and P=0.27 for non-linearity, respectively). The summary relative risk of coronary heart disease for an increase of one egg consumed per day was 0.99 (95% confidence interval 0.85 to 1.15; P=0.88 for linear trend) without heterogeneity among studies (P=0.97, I2=0%). For stroke, the combined relative risk for an increase of one egg consumed per day was 0.91 (0.81 to 1.02; P=0.10 for linear trend) without heterogeneity among studies (P=0.46, I2=0%). In a subgroup analysis of diabetic populations, the relative risk of coronary heart disease comparing the highest with the lowest egg consumption was 1.54 (1.14 to 2.09; P=0.01). In addition, people with higher egg consumption had a 25% (0.57 to 0.99; P=0.04) lower risk of developing hemorrhagic stroke.
Conclusions Higher consumption of eggs (up to one egg per day) is not associated with increased risk of coronary heart disease or stroke. The increased risk of coronary heart disease among diabetic patients and reduced risk of hemorrhagic stroke associated with higher egg consumption in subgroup analyses warrant further studies.
Introduction
Cardiovascular disease is now a public health crisis, affecting millions of people in both developed and developing countries. Although the rate of death attributable to the disease has declined in developed countries in the past several decades, it is still the leading cause of death and extorts a heavy social and economic toll globally.1 2 3 In low and middle income countries, the prevalence of cardiovascular disease has increased dramatically. By 2020, the disease is forecasted to be the major cause of morbidity and mortality in most developing nations.4
In recent decades, concern has mounted regarding the high prevalence and costs associated with cardiovascular disease, with growing interest in altering risk factors and reversing this global epidemic. Among the known risk factors for cardiovascular disease, levels of low density lipoprotein (LDL) cholesterol have aroused particular attention. In the Women’s Health Study, after a mean follow-up of eight years, participants with the highest levels of LDL cholesterol showed a notably higher risk of cardiovascular events than those with the lowest levels.5 In addition, several meta-analyses of observational studies and randomized controlled trials have found that a reduction in concentrations of LDL cholesterol could significantly reduce the risk of coronary heart disease and stroke incidence and mortality.6 7 8 9 Diet is an important determinant of serum cholesterol, but dietary cholesterol has only a modest contribution to plasma concentrations of LDL cholesterol.10 On the other hand, dietary cholesterol may prompt the oxidation of LDL and increase postprandial lipemia, which could raise the risk of vascular disease.11 To minimize the elevation of blood cholesterol and reduce the risk of cardiovascular disease, the American Heart Association (AHA) has recommended the public to consume less than 300 mg/day of cholesterol.12 13
Since eggs are a major source of dietary cholesterol, with one large egg containing almost 210 mg of cholesterol, the public has been recommended to limit egg consumption unless the intake of other foods high in cholesterol is restricted.14 However, eggs are also an inexpensive and low calorie source of many other nutrients, including minerals, proteins, and unsaturated fatty acids, which could lower the risk of cardiovascular disease.15 Additionally, in populations following a carbohydrate restricted diet, dietary cholesterol from eggs could increase plasma concentrations of high density lipoprotein (HDL) cholesterol,16 which has been suggested to protect against vascular disease.17 18 Therefore, some organizations have recommended that reducing egg intake might not be important for healthy people with normal levels of cholesterol in the blood.19 Food based dietary guidelines from countries including Nepal, Thailand, and South Africa recommend consuming eggs every day or regularly as part of a healthy diet.20
Several prospective cohort studies have examined the association between egg consumption and risk of coronary heart disease and stroke. However, the relation between egg consumption and risk of cardiovascular disease remains controversial. Therefore, we conducted a dose-response meta-analysis of prospective cohort studies to quantify the association between egg consumption and risk of coronary heart disease and stroke.
Methods
Search strategy
We conducted a literature search of PubMed (Medline) and Embase from January 1966 through June 2012 for prospective cohort studies examining the association between egg consumption and risk of CHD and stroke. PubMed search terms were “Cardiovascular Diseases”[MeSH] or “Stroke”[MeSH] or “Coronary Disease”[MeSH] or “myocardial infarction”[MeSH] or CHD) and egg. Similar search terms were used for Embase. In addition, we scrutinized references from relevant original papers and review articles to identify further pertinent studies. No language restrictions were imposed. We followed the standard criteria for conducting meta-analyses of observational studies and reporting the results.21
Study selection
Studies were included in this meta-analysis if they satisfied the following criteria: the study design was prospective, the exposure of interest was egg consumption, the outcome was coronary heart disease or stroke, and the investigators reported relative risks with 95% confidence intervals for at least three quantitative categories of egg intake. Additionally, we excluded reviews, editorials, non-human studies, and letters without sufficient data. Studies of other exposures and diseases were also excluded. If study populations were reported more than once, we used the result with the longest follow-up time.
Data extraction
Data extraction was carried out independently by two authors (YR and LC) using a standard extraction form. We extracted the following information from each study: authors, year of publication, study name, study location, years of follow-up, sample size (number of participants and incident cases), participants’ characteristics (age and sex), endpoints (coronary heart disease, stroke, or both), outcomes ascertainment, egg consumption categories, covariates adjusted in the multivariable analysis, and relative risks (95% confidence intervals) for all categories of egg consumption.
Quality assessment was performed according to the Newcastle-Ottawa quality assessment scale,22 which is a validated scale for non-randomised studies in meta-analyses. This scale awards a maximum of nine points to each study: four for selection of participants and measurement of exposure, two for comparability of cohorts on the basis of the design or analysis, and three for assessment of outcomes and adequacy of follow-up. We assigned scores of 0-3, 3.5-6, and 6.5-9 for low, moderate, and high quality of studies, respectively. When studies had several adjustment models, we extracted those that reflected the maximum extent of adjustment for potentially confounding variables.
For studies that reported egg intake as servings per week or day, we assumed that each serving was equivalent to one egg. For studies that lacked the unit of consumption, the categories were estimated by multiplying the frequency of consumption from the food frequency questionnaires with an average portion size according to the mean intake derived from the 24 h diaries. We contacted the authors if the data of interest were not directly shown in the publications. To resolve discrepancies, we used group consensus and consulted a third reviewer.
Statistical analysis
In this meta-analysis, the relative risks and 95% confidence intervals were considered as the effect size for all studies, and the hazard ratios were deemed equivalent to relative risks. Any results stratified by sex were treated as two separate reports. Those articles reporting both coronary heart disease and stroke were also treated as two separate reports. Owing to the distinct cut-off points for categories in different articles, we computed a relative risk with 95% confidence interval for an increased intake of one egg per day for each report. The method described by Greenland and Longnecker23 and Orsini and colleagues24 was used to calculate the trend from the correlated estimates for log relative risk across categories of egg consumption. The amount of egg consumption, distributions of cases and person years, and relative risks and 95% confidence intervals were extracted according to this method.
The median or mean egg consumption in each category was used as the corresponding dose of consumption. The midpoint of the upper and lower boundaries was considered the dose of each category if the median or mean intake per category was not available. If the highest category was open ended, the midpoint of the category was set at 1.5 times the lower boundary. If the number of cases and person years were not available, we used the relative risks comparing the highest versus lowest categories of egg intake to obtain a summary estimate.
In addition, we evaluated a potential curve linear association between egg consumption and risk of coronary heart disease and stroke, using restricted cubic splines with three knots at percentiles 10%, 50%, and 90% of the distribution.25 A P value for curve linearity or non-linearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to zero.
The heterogeneity among studies was estimated by the Cochran Q test and I2 statistic.26 Heterogeneity was confirmed with a significance level of P≤0.10. The I2 statistic describes the percentage of total variation in point estimates that can be attributed to heterogeneity. For the I2 metric, we considered low, moderate, and high I2 values to be 25%, 50%, and 75%, respectively.26 27 We used a fixed effect model (Mantel-Haenszel method) when heterogeneity was negligible, and a random effect model (DerSimonian and Laird method) when heterogeneity was significant.28 Forest plots and funnel plots were used to examine the overall effect and assess the publication bias, respectively.
We also conducted analyses stratified by sex, study location, number of cases and participants, duration of follow-up, repeated egg consumption measurements, study quality, and whether diet variables or cholesterol levels were controlled for in models. All statistical analyses were performed with Stata version 11 (Stata Corp), and all tests were two sided with a significance level of 0.05.
Results
Literature search
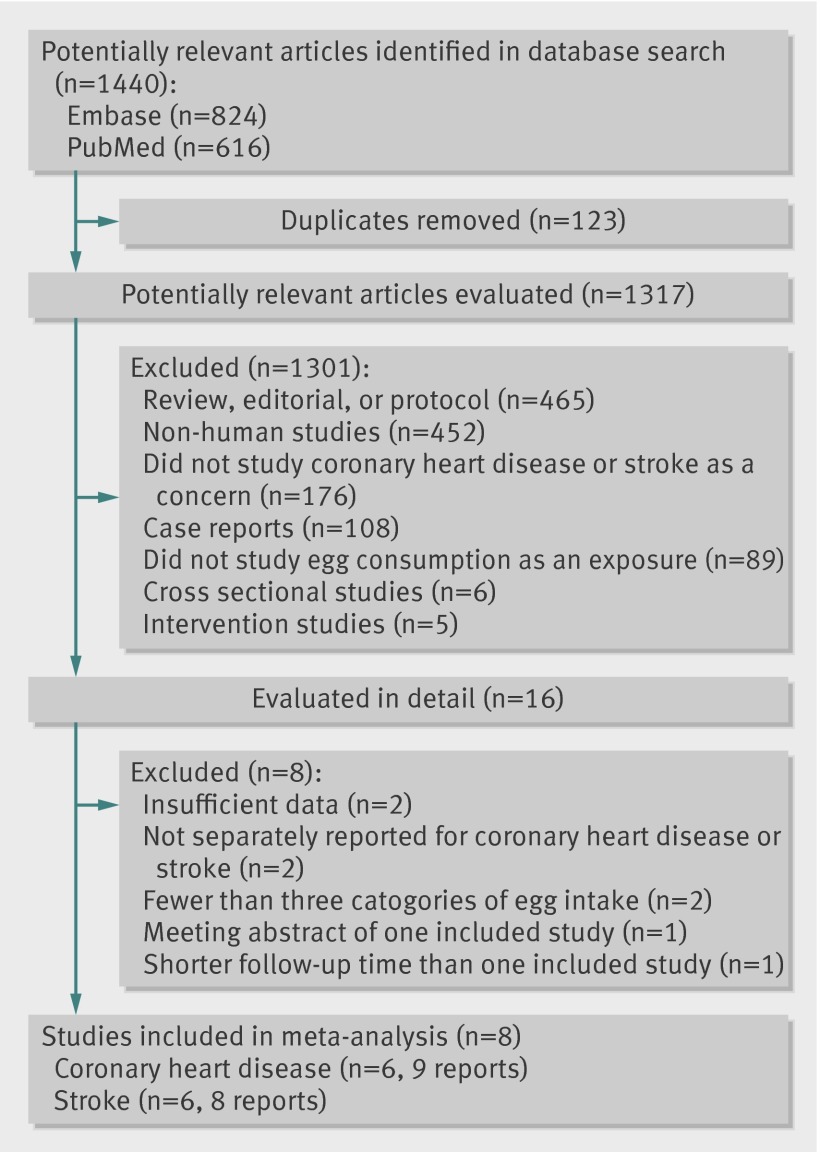
Figure 1 shows the results of literature research and selection. We identified 616 articles from PubMed and 824 articles from Embase prior to 20 June 2012. After exclusion of duplicates and studies that did not fulfill the inclusion criteria, 16 remaining articles seemed to be relevant for this meta-analysis. After evaluating the full texts of these 16 publications, we excluded eight articles as follows. Two articles29 30 were excluded owing to lack of sufficient data for estimation of relative risks. Another two articles31 32 were excluded because they did not separately report the relative risks and 95% confidence intervals for coronary heart disease or stroke. We also excluded one report33 because it was the meeting abstract of the study by Scrafford and colleagues.34 A study by He and colleagues35 was excluded because it reported the intermediate follow-up results of Health Professionals Follow-up Study. The final meta-analysis included eight articles, of which four34 36 37 38 examined men and women separately. For the study by Scrafford and colleagues,34 the estimate of the association between egg consumption and stroke mortality among men was imprecise because of sparse data, and thus for this report we included only data for women. In total, our meta-analysis included eight articles with 17 independent reports.
Fig 1 Flow diagram of literature search and study selection
Study characteristics
Tables 1 and 2 show the information extracted from the included studies, all of which had prospective cohort designs and participants with no prior diagnoses of cardiovascular disease at baseline. The meta-analysis consisted of 263 938 participants with 3 081 269 person years of follow-up for coronary heart disease, and 210 404 patients with 4 148 095 person years of follow-up for stroke. Among the participants, we documented 5847 cases of coronary heart disease during follow-up periods ranging from eight to 20 years, and 7579 cases of stroke during a follow-up ranging from 8.8 to 22 years. Three cohorts37 39 40 were among Asians (Japan), and the others34 36 38 41 42 were conducted in the United States. Egg consumption was measured by food frequency questionnaires in all studies. Four studies36 38 40 42 used repeated measurements to update dietary information to more accurately reflect the dietary intakes over follow-up. Results of study quality assessment (score 0-9) yielded a score of 6.5 or above (high quality) for all studies, with an average score of 7.6 (web appendix, tables A and B).
Table 1.
Characteristics of participants and follow-up in included studies of egg consumption in relation to risk of coronary heart disease and stroke
| Author | Publication year | Study name | Country | Sex of population | Age at baseline (years) | No of participants | Mean length of follow-up (years) | Endpoints (No of cases) |
|---|---|---|---|---|---|---|---|---|
| Hu et al36 | 1999 | Health Professionals Follow-up Study | USA | Male | 40-75 | 37 851 | 8 | Coronary heart disease (866) |
| Hu et al36 | 1999 | Nurses’ Health Study | USA | Female | 34-59 | 80 082 | 14 | Coronary heart disease (939) |
| Sauvaget et al39 | 2003 | Hiroshima/Nagasaki Life Span Study | Japan | Male and female | 34-103 | 34 807 | 16-17 | Stroke mortality (1259) |
| Nakamura et al37 | 2004 | NIPPON DATA80 | Japan | Male | 30+ | 4077 | 14 | Ischemic heart disease mortality (39), stroke mortality (112) |
| Nakamura et al37 | 2004 | NIPPON DATA80 | Japan | Female | 30+ | 5186 | 14 | Ischemic heart disease mortality (41), stroke mortality (107) |
| Nakamura et al40 | 2006 | Japan Public Health Center-based prospective study | Japan | Male and female | 40-69 | 90 735 | 10.2 | Coronary heart disease (462) |
| Qureshi et al41 | 2007 | NHANES-I | USA | Male and female | 25-74 | 9734 | 20 | Coronary artery disease (1584), stroke (655) |
| Djousse et al42 | 2008 | Physicians’ Health Study I | USA | Male | 40-85 | 21 327 | 20 | Myocardial infarction (1550), stroke (1342) |
| Scrafford et al34 | 2011 | NHANES III | USA | Male | 17+ | 6833 | 8.8 | Coronary heart disease mortality (198), stroke mortality (63) |
| Scrafford et al34 | 2011 | NHANES III | USA | Female | 17+ | 8113 | 8.9 | Coronary heart disease mortality (168), stroke mortality (74) |
| Bernstein et al38 | 2012 | Health Professionals Follow-Up Study | USA | Male | 40-75 | 43 150 | 22 | Stroke (1397) |
| Bernstein et al38 | 2012 | Nurses’ Health Study | USA | Female | 30-55 | 84 010 | 26 | Stroke (2633) |
NHANES-I=First National Health and Nutrition Examination Survey; NHANES III=Third National Health and Nutrition Examination Survey; NIPPON DATA80=National Integrated Project for Prospective Observation of Non-communicable Disease and its Trends in the Aged, 1980.
Table 2.
Outcomes and covariates of included studies of egg consumption in relation to risk of coronary heart disease and stroke
| Study | Endpoints | Case ascertainment | Dietary category and relative risk (95% CI) | Covariates in fully adjusted model |
|---|---|---|---|---|
| Hu et al, 199936 (men) | Coronary heart disease | Self reported diagnosis or confirmed by medical records or autopsy | <1 egg/week, 1.0 (reference); 1, 1.06 (0.88 to 1.27); 2-4, 1.12 (0.95 to 1.33); 5-6, 0.90 (0.63 to 1.27); ≥7, 1.08 (0.79 to 1.48) | Age, body mass index, two year time periods, smoking, parental history of myocardial infarction, multivitamin supplement use, alcohol consumption, menopausal status and postmenopausal hormone use (women), history of hypertension, physical activity, and total energy intake |
| Hu et al, 199936 (women) | Coronary heart disease | Same as above | <1 egg/week, 1.0 (reference); 1, 0.82 (0.67 to 1.00); 2-4, 0.99 (0.82 to 1.18); 5-6, 0.95 (0.70 to 1.29); ≥7, 0.82 (0.60 to 1.13) | |
| Sauvaget et al, 200339 | Stroke mortality | Confirmed by nationwide family registration system | Never, 1.0 (reference); ≤1 egg/week*, 0.74 (0.55 to 1.00); 2-4 eggs/week, 0.78 (0.59 to 1.04); almost daily, 0.72 (0.54 to 0.97) | Age, sex, birth cohort, city, radiation dose, body mass index, smoking, alcohol habits, education level, history of diabetes, or hypertension |
| Nakamura et al, 200437 (men) | Ischemic heart disease mortality, stroke mortality | Confirmed by National Vital Statistics | Ischemic heart disease mortality: seldom, 1.18 (0.26 to 5.42); 1-2 eggs/week, 1.71 (0.78 to 3.76); 0.5 eggs/day, 1.49 (0.63 to 3.48); 1 egg/day, 1.0 (reference) Stroke mortality: seldom, 0.93 (0.36 to 2.40); 1-2 eggs/week, 1.09 (0.69 to 1.72); 0.5 eggs/day, 1.10 (0.68 to 1.76); 1 egg/day, 1.0 (reference); ≥2 eggs/day, 0.25 (0.03 to 1.81) |
Age, serum creatinine, total cholesterol, blood glucose, body mass index, blood pressures, use of blood pressure lowering drugs, smoking, and alcohol intake |
| Nakamura et al, 200437 (women) | Ischemic heart disease mortality, stroke mortality | Same as above | Ischemic heart disease mortality: seldom, 1.42 (0.56 to 3.62); 1-2 eggs/week, 0.64 (0.28 to 1.44); 0.5 eggs/day, 0.78 (0.35 to 1.82); 1 egg/day, 1.0 (reference); ≥2 eggs/day, 1.27 (0.16 to 9.80) Stroke mortality: seldom, 0.78 (0.35 to 1.73); 1-2 eggs/week, 0.79 (0.47 to 1.33); 0.5 eggs/day, 1.46 (0.89 to 2.4); 1 egg/day, 1.0 (reference); ≥2 eggs/day, 1.22 (0.29 to 5.17) |
|
| Nakamura et al, 200640 | Coronary heart disease | Confirmed by medical records, letter, telephone or death certificate | <1 egg/week*, 1.19 (0.86 to 1.64); 1-2 eggs/week, 1.00 (0.77 to 1.30); 3-4 eggs/week, 1.00 (0.79 to 1.26); almost daily, 1.0 (reference) | Age; sex; body mass index; hypertension; diabetes; use of cholesterol lowering drugs; smoking; alcohol drinking; whether participants intended to avoid cholesterol rich diets; consumption frequencies of meat, fish, vegetables, and fruits; and cohort effects |
| Qureshi et al, 200741 | Coronary artery disease, stroke | Confirmed by medical records or death certificate | Coronary artery disease: <1 egg/week, 1.0 (reference); 1-6, 1.0 (0.9 to 1.1); >6, 1.1 (0.9 to 1.3) Stroke: <1 egg/week, 1.0 (reference); 1-6, 0.9 (0.7 to 1.0); >6, 0.9 (0.7 to 1.1) |
Age, sex, race or ethnicity, systolic blood pressure, diabetes mellitus, serum cholesterol, smoking, body mass index, and educational status |
| Djousse et al, 200842 | Myocardial infarction, stroke | Confirmed by physicians or medical records | Myocardial infarction: <1 egg/week, 1.0 (reference); 1, 1.12 (0.96 to 1.31); 2-4, 1.16 (1.00 to 1.36); 5-6, 1.18 (0.93 to 1.49); ≥7, 0.90 (0.72 to 1.14) Stroke: <1 egg/week, 1.0 (reference); 1, 0.96 (0.82 to 1.13); 2-4, 1.06 (0.91 to 1.24); 5-6, 1.13 (0.89 to 1.42); ≥7, 0.99 (0.80 to 1.23) |
Age, body mass index, smoking, history of hypertension, vitamin intake, alcohol consumption, vegetable consumption, breakfast cereal, physical activity, treatment arm, atrial fibrillation, diabetes mellitus, hypercholesterolemia, and parental history of premature myocardial infarction |
| Scrafford et al, 201134 (men) | Coronary heart disease mortality, stroke mortality | Not applicable | Coronary heart disease mortality: <1 egg/week, 1.0 (reference); 1-6, 1.26 (0.79 to 2.00); >6, 1.13 (0.61 to 2.11) Stroke mortality: <1 egg/week, 1.0 (reference); 1-6, 1.00 (0.49 to 2.02); >6, 0.27 (0.10 to 0.73) |
Age, energy, marital status, educational status, race or ethnicity, smoking, body mass index, waist to hip ratio, diabetes, hypertension, and dietary variables |
| Scrafford et al, 201134 (women) | Coronary heart disease mortality, stroke mortality | Not applicable | Coronary heart disease mortality: <1 egg/week, 1.0 (reference); 1-6, 1.12 (0.66 to 1.89); >6, 0.92 (0.27 to 3.11) Stroke mortality: <1 egg/week, 1.0 (reference); 1-6, 0.93 (0.46 to 1.90); >6, 1.03 (0.25 to 4.22) |
|
| Bernstein et al, 201238 (men) | Stroke | Confirmed by medical records or autopsy report | 0.14 eggs/week†, 1.0 (reference); 0.49, 0.80 (0.66 to 0.97); 1.19, 0.88 (0.73 to 1.05); 3.01, 0.80 (0.66 to 0.96); 5.53, 0.84 (0.68 to 1.04) | Age, time period, body mass index, smoking, physical exercise, parental history of early myocardial infarction, menopausal status in women, multivitamin use, vitamin E supplement use, aspirin use at least once per week, total energy, cereal fiber, alcohol, trans fat, fruit and vegetables, and other protein sources |
| Bernstein et al, 201238 (women) | Stroke | Same as above | 0.49 eggs/week†, 1.0 (reference); 1.26, 0.90 (0.80 to 1.01); 2.17, 0.94 (0.83 to 1.05); 3.01, 0.86 (0.76 to 0.99); 4.69, 0.91 (0.80 to 1.04) |
*Unit of egg consumption was assumed.
†Servings per day converted to eggs consumed per week.
Association between egg consumption and risk of coronary heart disease
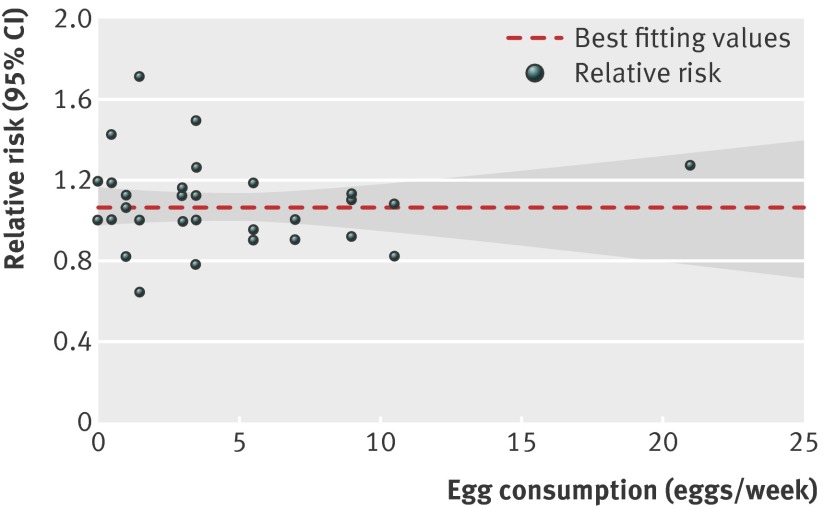
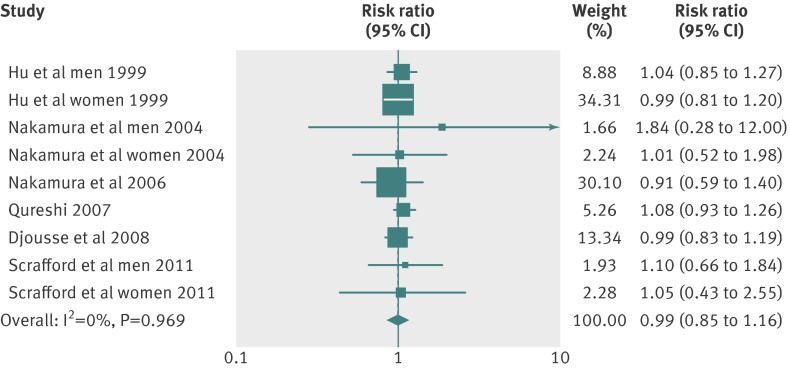
Six articles34 36 37 40 41 42 with nine reports were included in the dose-response analysis of egg consumption and risk of coronary heart disease. Using a restricted cubic splines model, we found no evidence of a curve linear association between egg consumption and risk of coronary heart disease (P=0.67 for non-linearity; fig 2). The summary relative risk of coronary heart disease for an increase of one egg per day was 0.99 (95% confidence interval 0.85 to 1.15; P=0.88 for linear trend). We saw no heterogeneity among studies (P=0.97, I2=0%; fig 3). Additionally, Begg and Egger regression tests provided no evidence of substantial publication bias (P>0.05 for both tests). Among the included studies, two articles34 37 (four total reports) examined the relation between egg consumption and risk of coronary heart disease in populations with diabetes. Owing to the lack of data for person years in diabetic populations, we obtained the summary relative risk comparing the highest with the lowest egg consumption for coronary heart disease in diabetic patients (relative risk 1.54 (1.14 to 2.09); P=0.01; table 3; web appendix, table C).
Fig 2 Dose-response analyses of egg consumption and risk of coronary heart disease
Fig 3 Forest plot of egg consumption and risk of coronary heart disease
Table 3.
Stratified analyses of relative risk of coronary heart disease and stroke
| No of reports* | Relative risk (95% CI) | P for heterogeneity | I2 | P for test | |
|---|---|---|---|---|---|
| Coronary heart disease | |||||
| Total cases | 9 | 0.99 (0.85 to 1.15) | 0.97 | 0.0 | 0.88 |
| Fatal cases | 4 | 1.18 (0.71 to 1.96) | 0.91 | 0.0 | 0.53 |
| Coronary heart disease with diabetes† | 5 | 1.54 (1.14 to 2.09) | 0.59 | 0.0 | 0.01 |
| Subgroup analyses for total coronary heart disease | |||||
| Sex | |||||
| Male | 4 | 1.06 (0.89 to 1.25) | 0.83 | 0.0 | 0.53 |
| Female | 3 | 0.99 (0.83 to 1.20) | 0.99 | 0.0 | 0.95 |
| Study location | |||||
| USA | 6 | 1.01 (0.90 to 1.13) | 0.96 | 0.0 | 0.89 |
| Asia | 3 | 0.95 (0.64 to 1.41) | 0.76 | 0.0 | 0.79 |
| No of participants | |||||
| >10 000 | 4 | 0.97 (0.81 to 1.15) | 0.87 | 0.0 | 0.70 |
| ≤10 000 | 5 | 1.14 (0.83 to 1.56) | 0.93 | 0.0 | 0.42 |
| No of cases | |||||
| >500 | 4 | 1.00 (0.89 to 1.13) | 0.79 | 0.0 | 0.94 |
| ≤500 | 5 | 0.96 (0.67 to 1.37) | 0.93 | 0.0 | 0.83 |
| Duration of follow-up | |||||
| >15 years | 2 | 1.02 (0.89 to 1.16) | 0.40 | 0.0 | 0.83 |
| ≤15 years | 7 | 0.98 (0.81 to 1.19) | 0.98 | 0.0 | 0.85 |
| Repeated egg consumption measurements | |||||
| Yes | 4 | 0.97 (0.81 to 1.15) | 0.87 | 0.0 | 0.70 |
| No | 5 | 1.14 (0.83 to 1.56) | 0.93 | 0.0 | 0.42 |
| Study quality | |||||
| Score ≥8 | 5 | 0.97 (0.80 to 1.17) | 0.93 | 0.0 | 0.74 |
| Score <8 | 4 | 1.06 (0.88 to 1.28) | 0.81 | 0.0 | 0.55 |
| Controlling for other diet variables in models‡ | |||||
| Yes | 6 | 0.97 (0.82 to 1.15) | 0.97 | 0.0 | 0.73 |
| No | 5 | 1.14 (0.84 to 1.55) | 0.92 | 0.0 | 0.40 |
| Controlling for serum cholesterol levels or use of cholesterol lowering drugs in models | |||||
| Yes | 5 | 0.97 (0.75 to 1.26) | 0.65 | 0.0 | 0.83 |
| No | 4 | 1.01 (0.86 to 1.18) | 0.97 | 0.0 | 0.94 |
| Stroke | |||||
| Total stroke | 8 | 0.91 (0.81 to 1.02) | 0.46 | 0.0 | 0.10 |
| Fatal stroke | 4 | 0.94 (0.81 to 1.10) | 0.47 | 0.0 | 0.46 |
| Hemorrhagic stroke† | 3 | 0.75 (0.57 to 0.99) | 0.21 | 36.8 | 0.04 |
| Ischemic stroke† | 4 | 0.91 (0.82 to 1.01) | 0.79 | 0.0 | 0.08 |
| Stroke with diabetes† | 3 | 0.80 (0.29 to 2.15) | 0.09 | 58.9 | 0.65 |
| Subgroup analyses for total stroke | |||||
| Sex | |||||
| Male | 4 | 0.89 (0.77 to 1.03) | <0.001 | 90.5 | 0.13 |
| Female | 4 | 0.91 (0.77 to 1.07) | 0.24 | 29.0 | 0.25 |
| Study location | |||||
| USA | 5 | 0.90 (0.79 to 1.03) | 0.38 | 4.9 | 0.13 |
| Asia | 3 | 0.94 (0.83 to 1.07) | 0.28 | 20.6 | 0.34 |
| No of participants | |||||
| >10 000 | 4 | 0.90 (0.80 to 1.02) | 0.25 | 26.9 | 0.10 |
| ≤10 000 | 4 | 0.97 (0.75 to 1.27) | 0.50 | 0.0 | 0.85 |
| No of cases | |||||
| >500 | 5 | 0.91 (0.81 to 1.02) | 0.38 | 5.0 | 0.09 |
| ≤500 | 3 | 1.00 (0.63 to 1.59) | 0.35 | 4.2 | 0.99 |
| Duration of follow-up | |||||
| >15 years | 5 | 0.91 (0.81 to 1.02) | 0.38 | 5.0 | 0.09 |
| ≤15 years | 3 | 1.00 (0.63 to 1.59) | 0.35 | 4.2 | 0.99 |
| Repeated egg consumption measurements | |||||
| Yes | 3 | 0.90 (0.78 to 1.04) | 0.13 | 51.5 | 0.14 |
| No | 5 | 0.94 (0.83 to 1.08) | 0.64 | 0.0 | 0.38 |
| Study quality | |||||
| Score ≥7.5 | 5 | 1.03 (0.88 to 1.20) | 0.51 | 0.0 | 0.71 |
| Score <7.5 | 3 | 0.88 (0.77 to 1.01) | 0.82 | 0.0 | 0.07 |
| Controlling for other diet variables in models‡ | |||||
| Yes | 4 | 0.90 (0.79 to 1.04) | 0.25 | 26.6 | 0.14 |
| No | 5 | 0.95 (0.83 to 1.08) | 0.62 | 0.0 | 0.43 |
| Controlling for serum cholesterol levels or use of cholesterol lowering drugs in models | |||||
| Yes | 4 | 1.04 (0.91 to 1.18) | 0.34 | 10.0 | 0.60 |
| No | 4 | 0.89 (0.77 to 1.01) | 0.94 | 0.0 | 0.07 |
*Five articles reported their results by sex group; therefore, there were nine reports from six articles for coronary heart disease and eight reports from six articles for stroke.
†Owing to a lack of data for person years, results are relative risks (95% confidence intervals) comparing highest with lowest egg consumption.
‡Study by Scrafford and colleagues reported results both controlling for diet variables and not.
Association between egg consumption and risk of stroke
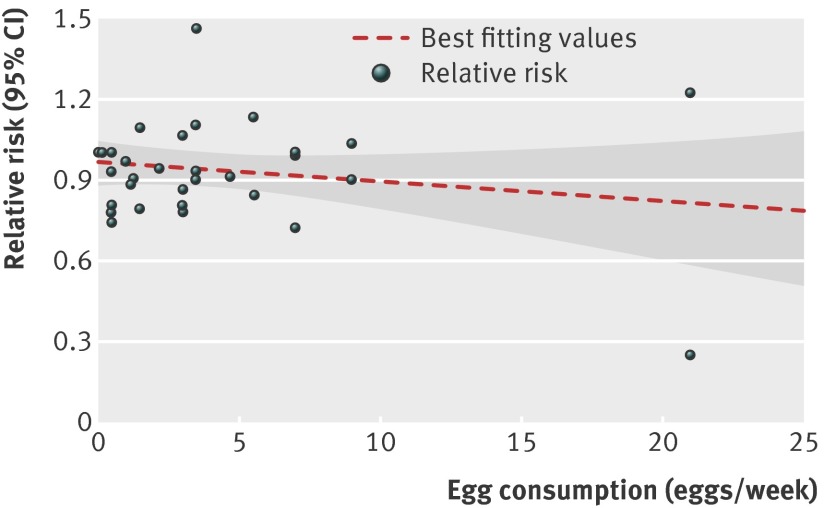
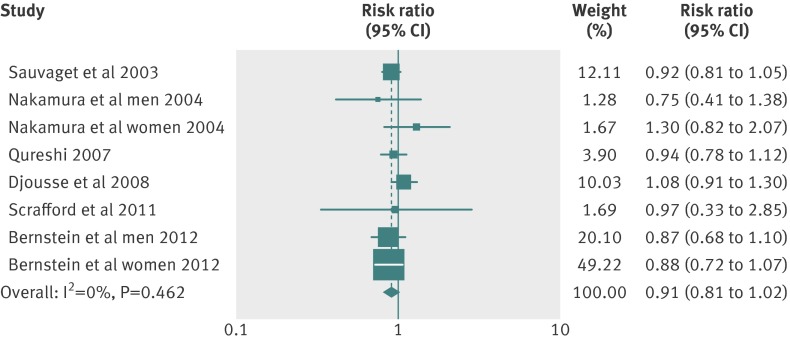
This dose-response analysis involved six articles34 37 38 39 41 42 with eight reports on egg consumption and stroke risk. We did not find a curve linear association between egg consumption and risk of stroke (P=0.27 for non-linearity; fig 4). The combined relative risk of stroke for an increment of one egg consumed per day was 0.91 (95% confidence interval 0.81 to 1.02; P=0.10 for linear trend; fig 5). No heterogeneity of effect estimates on relative risks was observed (P=0.46, I2=0%). Neither the Begg test nor the Egger test for publication bias reached significance (P>0.05 for both tests). In addition, three articles34 37 39 with four reports provided information on fatal stroke (pooled relative risk 0.94 (0.80 to 1.10); P=0.46; table 3). Moreover, four articles38 39 41 42 reported results for different types of stroke, and three articles34 41 42 provided results for stroke in those with diabetes. For these studies, the combined relative risks comparing the highest versus lowest egg intake were 0.75 (0.57 to 0.99) for hemorrhagic stroke, 0.91 (0.82 to 1.01) for ischemic stroke, and 0.80 (0.29 to 2.15) for total stroke among people with diabetes (table 3; web appendix, table D).
Fig 4 Dose-response analyses of egg consumption and risk of stroke
Fig 5 Forest plot of egg consumption and risk of stroke
Subgroup analyses
Subgroup analyses were conducted to examine the stability of the primary results (table 3). The associations between egg consumption and risk of coronary heart disease and stroke were similar in subgroup analyses, which were defined by sex, study location, number of cases or participants, duration of follow-up, repeated egg consumption measurements, study quality, and whether diet variables or cholesterol levels were controlled for in models. An increment of one egg consumed per day did not significantly increase risk of coronary heart disease or stroke in any of the categories.
Discussion
This meta-analysis identified no significant association between egg consumption and risk of coronary heart disease or stroke. Higher intake of eggs (up to one egg per day) was not associated with risk of coronary heart disease or stroke. Similar results were obtained in subgroup analyses. However, among diabetic participants, higher egg consumption was associated with a significantly elevated risk of coronary heart disease. On the other hand, higher egg intake was associated with a lower risk of hemorrhagic stroke. These subgroup results should be interpreted with caution, because only a few studies focused on diabetic participants and particular stroke subtypes.
Results in relation to other studies
To date, the majority of prospective studies have found no significant association between egg consumption and risk of coronary heart disease or stroke. However, Burke and colleagues43 analyzed data from 514 Western Australian aborigines with almost 14 years of follow-up and found that risk of coronary heart disease increased in participants consuming eggs more than twice per week. But this study was small and analyzed multiple dietary and lifestyle exposures.
Some studies have found an inverse association between egg consumption and stroke risk. For example, an analysis of the Third National Health and Nutrition Examination Survey 1988-1994 (NHANES III) dataset found a significant inverse association between higher egg consumption and stroke mortality among men.34 A cohort study from Japan found that increased consumption of animal products (including eggs) was associated with reduced risk of total and hemorrhagic stroke death.39
We considered several potential reasons for the lack of an overall association between egg consumption and coronary heart disease or stroke. Although dietary cholesterol influences plasma concentrations of serum cholesterol, the effects are relatively small.10 In addition, epidemiologic studies have found weak or little association between dietary cholesterol intake and cardiovascular disease risk.10 Apart from dietary cholesterol, saturated fat and dietary patterns might also influence blood cholesterol levels,44 45 46 suggesting that compliance with general dietary recommendations instead of simply reducing egg consumption could have a greater effect on the risk of cardiovascular disease. Additionally, individual differences in response to dietary cholesterol vary greatly, which could affect the association between egg consumption and risk of coronary heart disease and stroke. Moreover, several studies have shown that egg consumption favors the formation of larger LDL and HDL particles, which might enhance protection against atherosclerosis.47 48
Other than cholesterol, eggs are a good source of other nutrients such as high quality protein and vitamin D. In the Diet, Obesity, and Gene (Diogenes) Project, increased protein consumption together with a modest reduction in glycemic index was beneficial for weight control.49 Substituting protein for carbohydrate also partly resulted in lower blood pressure, improved lipids levels, and concomitantly reduced cardiovascular risk.50 Higher vitamin D intake might have beneficial effects on the reduction of visceral adipose tissue51 and other cardiovascular risk factors52.
Another possibility is that lifestyle factors associated with egg consumption might have obscured a positive association between egg consumption and risk of coronary heart disease and stroke. However, regular egg consumption tends to be associated with unhealthy lifestyle factors such as smoking and physical inactivity.34 36 53 Higher consumption of eggs is also likely to be associated with increased consumption of red and processed meats.36 These confounding factors tend to exaggerate rather than mask the association between egg consumption and cardiovascular disease risk. One study found that participants with high levels of cholesterol in the blood were more likely to reduce their egg consumption than others.40 However, our subgroup analysis showed that the association between egg consumption and coronary heart disease was similar in the models, with or without adjustment for cholesterol levels.
Recently, a cross sectional study assessed the total plaque area in patients attending Canadian vascular prevention clinics to determine whether the atherosclerosis burden was related to dietary egg intake.54 The study found a strong positive association between the number of egg yolks and the degree of atherosclerosis measured by plaque areas. However, the study did not assess or adjust for other dietary or lifestyle factors and did not examine hard cardiovascular disease endpoints. The cross sectional nature of the study also limited causal interpretation of the data. Therefore, the results from this cross sectional analysis should be interpreted with caution.55 The findings from our meta-analyses of prospective cohort studies do not support a positive association between egg consumption and cardiovascular disease outcomes in the general population.
Subgroup analyses have suggested a positive association between egg consumption and coronary heart disease risk in diabetic patients. Among diabetic populations, decreased plasma levels of apolipoprotein E, together with increased levels of apolipoprotein C-III could lead to abnormal cholesterol transport, which might increase the risk of coronary heart disease.56 57 The adverse effect of egg consumption on lipoprotein profile and glycemic control could contribute to the elevated risk of coronary heart disease in diabetic populations.
In addition, insulin sensitivity could influence HDL metabolism and cholesterol transport.58 59 Riemens and colleagues60 found that people with lower insulin sensitivity had increased levels of plasma cholesterol, very low density lipoprotein cholesterol, and LDL cholesterol, compared with those with higher insulin sensitivity. Activities of plasma lecithin, cholesterol acyl transferase, phospholipid transfer protein, and hepatic lipase were negatively correlated with insulin sensitivity, which could have enhanced reverse cholesterol transport.60 These findings suggest a biological mechanism for possible adverse effects of insulin resistance on risk of coronary heart disease in diabetic populations through cholesterol metabolism. Nonetheless, this subgroup finding of a positive association between egg consumption and coronary heart disease risk was based on a small number of studies and thus needs to be replicated in further studies.
Several prospective cohort studies showed that hemorrhagic stroke had an inverse association with serum levels of cholesterol.61 62 63 64 In particular, the result of a meta-analysis including 13 cohorts from China and Japan showed that decreased cholesterol concentrations conferred an increased risk of hemorrhagic stroke.65 It has been suggested that low cholesterol levels promote necrosis of medial muscle cells and reduce platelet aggregability, which could lead to plasmatic arterionecrosis and the incidence of hemorrhagic stroke.66 67 It is unclear whether the inverse association between egg consumption and hemorrhagic stroke is mediated through low levels of serum cholesterol or other mechanisms. Since this subgroup finding was based on a small number of studies, the results should be interpreted with caution.
Strengths and limitations
Our study has several strengths. Our meta-analysis included prospective cohort studies with large sample size and long duration of follow-up, which significantly increased the statistical power to detect potential associations. We investigated a dose-response relation between egg consumption and risk of coronary heart disease and stroke, allowing us to examine the shape of this possible association. Linear and non-linear relations were also tested to quantify the associations. In addition, we used models adjusting for most established risk factors and did stratified analyses to explore whether some factors could explain the results.
Several limitations of our study should also be acknowledged. Firstly, errors in measurement of egg intake and other dietary habits could have attenuated individual study results and led to the null association between egg consumption and risk of coronary heart disease and stroke. All the studies in our analysis assessed egg consumption using food frequency questionnaires, several of which have been validated with reasonable reproducibility and validity of self reported egg intake. However, misreporting of intake was still inevitable.68 69 70
The cooking methods of eggs and the amount of salt added to eggs were not available in most of the included studies. The nutrient contents of eggs could alter depending on different cooking methods or feeding methods of chicken. In addition, we could not uniformly quantify the size of eggs in each study. Moreover, participants with higher egg intake consumed more dietary cholesterol and protein but fewer carbohydrates and were more likely to have lower levels of education than those with lower egg intake.34 36 Several studies adjusted for those confounding factors. To reduce this bias, we conducted a stratified analysis and found the results to be robust in different strata of covariates.
Secondly, during the long follow-up, participants may have changed their diets. However, in our meta-analysis, nearly half the included studies updated the diet information from food frequency questionnaires. Stratified analysis indicated that the associations between egg consumption and risk of coronary heart disease and stroke were similar, regardless of whether repeated egg consumption measurements were considered.
Thirdly, some studies considered the intake of foods in which egg was the main ingredient. However, the results suggested that the amount of eggs estimated in other foods was relatively small and was unlikely to affect the aforementioned associations. Finally, the statistical power was limited in subgroup analyses of diabetic patients or subtypes of stroke.
Conclusions
In summary, results from our meta-analysis do not support that higher egg consumption is associated with elevated risk of coronary heart disease and stroke. Subgroup analyses suggest a positive association between higher egg intake and risk of coronary heart disease in diabetic patients, and an inverse association between higher egg consumption and incidence of hemorrhagic stroke. Studies with larger sample sizes and longer follow-up times are warranted to confirm these subgroup results.
What is already known on this topic
Cardiovascular disease affects millions of people in both developed and developing countries
As a major source of dietary cholesterol, eggs have been investigated by several epidemiologic studies in relation to risk of coronary heart disease and stroke
However, whether egg consumption increases the future risk of coronary heart disease and stroke remains unclear
What this study adds
Consumption of up to one egg per day was not associated with increased risk of coronary heart disease or stroke
Subgroup analysis suggested that consumption of up to one egg per day was associated with a significantly elevated risk of coronary heart disease in diabetic populations, and a reduced risk of hemorrhagic stroke
We thank Catherine Sauvaget, Eric J Grant, and Adam M Bernstein for providing data for the meta-analysis.
Contributors: YR and LL conceived the study. YR and LC searched the databases and checked them according to the eligible criteria and exclusion criteria. LL helped develop search strategies. TZ gave advice on meta-analysis methodology. YS helped extract quantitative data from some papers. YS, MY, and ZS analyzed the data. YR wrote the draft of the paper. LC, TZ, YS, MY, ZS, AS, FBH, and LL contributed to writing, reviewing, or revising the paper. LL is the guarantor.
Funding: This work was funded by the National Science and Technology Support Program (2012BAI02B02), National Natural Science Foundation (NSFC 81072291), and National Basic Research Program (2009CB118803) of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Ethical approval not needed.
Data sharing: No additional data available.
Cite this as: BMJ 2013;346:e8539
Web Extra. Extra material supplied by the author
Web appendix: Web tables
References
- 1.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol 2007;50:2128-32. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012;125:e2-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012;125:188-97. [DOI] [PubMed] [Google Scholar]
- 4.Celermajer DS, Chow CK, Marijon E, Anstey NM, Woo KS. Cardiovascular disease in the developing world: prevalences, patterns, and the potential of early disease detection. J Am Coll Cardiol 2012;60:1207-16. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002;347:1557-65. [DOI] [PubMed] [Google Scholar]
- 6.Cholesterol Treatment Trialists’ (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ 2009;338:b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes 2011;4:337-45. [DOI] [PubMed] [Google Scholar]
- 10.Kanter MM, Kris-Etherton PM, Fernandez ML, Vickers KC, Katz DL. Exploring the factors that affect blood cholesterol and heart disease risk: is dietary cholesterol as bad for you as history leads us to believe? Adv Nutr 2012;3:711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spence JD, Jenkins DJ, Davignon J. Dietary cholesterol and egg yolks: not for patients at risk of vascular disease. Can J Cardiol 2010;26:e336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arterioscler Thromb Vasc Biol 2006;26:2186-91. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114:82-96. [DOI] [PubMed] [Google Scholar]
- 14.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, et al. AHA Dietary Guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Stroke 2000;31:2751-66. [DOI] [PubMed] [Google Scholar]
- 15.Song WO, Kerver JM. Nutritional contribution of eggs to American diets. J Am Coll Nutr 2000;19(5 suppl):556-62S. [DOI] [PubMed] [Google Scholar]
- 16.Mutungi G, Ratliff J, Puglisi M, Torres-Gonzalez M, Vaishnav U, Leite JO, et al. Dietary cholesterol from eggs increases plasma HDL cholesterol in overweight men consuming a carbohydrate-restricted diet. J Nutr 2008;138:272-6. [DOI] [PubMed] [Google Scholar]
- 17.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huxley RR, Barzi F, Lam TH, Czernichow S, Fang X, Welborn T, et al. Isolated low levels of high-density lipoprotein cholesterol are associated with an increased risk of coronary heart disease: an individual participant data meta-analysis of 23 studies in the Asia-Pacific region. Circulation 2011;124:2056-64. [DOI] [PubMed] [Google Scholar]
- 19.Better Health Channel (Australia). Cholesterol. 2009. www.betterhealth.vic.gov.au/Bhcv2/bhcarticles.nsf/pages/Cholesterol_explained?open.
- 20.Food and Agricultural Organization of the United Nations. Food based dietary guidelines by country. 2009. www.fao.org/ag/humannutrition/nutritioneducation/fbdg/en/.
- 21.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [DOI] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2011. www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 23.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301-9. [DOI] [PubMed] [Google Scholar]
- 24.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal 2006;6:40-57. [Google Scholar]
- 25.Harrell FE Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 1988;80:1198-202. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol 2008;37:1158-60. [DOI] [PubMed] [Google Scholar]
- 28.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820-6. [DOI] [PubMed] [Google Scholar]
- 29.Dawber TR, Nickerson RJ, Brand FN, Pool J. Eggs, serum cholesterol, and coronary heart disease. Am J Clin Nutr 1982;36:617-25. [DOI] [PubMed] [Google Scholar]
- 30.Smith MA, Finn R, Green JR. Egg and meat consumption in myocardial infarction. Practitioner 1983;227:673-4. [PubMed] [Google Scholar]
- 31.Houston DK, Ding J, Lee JS, Garcia M, Kanaya AM, Tylavsky FA, et al. Dietary fat and cholesterol and risk of cardiovascular disease in older adults: the Health ABC Study. Nutr Metab Cardiovasc Dis 2011;21:430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zazpe I, Beunza JJ, Bes-Rastrollo M, Warnberg J, de la Fuente-Arrillaga C, Benito S, et al. Egg consumption and risk of cardiovascular disease in the SUN Project. Eur J Clin Nutr 2011;65:676-82. [DOI] [PubMed] [Google Scholar]
- 33.Scrafford C, Tran N, Barraj L. The impact of egg consumption on heart health using the NHANES III follow-up survey. FASEB J 2009;23(S1).
- 34.Scrafford CG, Tran NL, Barraj LM, Mink PJ. Egg consumption and CHD and stroke mortality: a prospective study of US adults. Public Health Nutr 2011;14:261-70. [DOI] [PubMed] [Google Scholar]
- 35.He K, Merchant A, Rimm EB, Rosner BA, Stampfer MJ, Willett WC, et al. Dietary fat intake and risk of stroke in male US healthcare professionals: 14 year prospective cohort study. BMJ 2003;327:777-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu FB, Stampfer MJ, Rimm EB, Manson JE, Ascherio A, Colditz GA, et al. A prospective study of egg consumption and risk of cardiovascular disease in men and women. JAMA 1999;281:1387-94. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura Y, Okamura T, Tamaki S, Kadowaki T, Hayakawa T, Kita Y, et al. Egg consumption, serum cholesterol, and cause-specific and all-cause mortality: the National Integrated Project for Prospective Observation of Non-communicable Disease and Its Trends in the Aged, 1980 (NIPPON DATA80). Am J Clin Nutr 2004;80:58-63. [DOI] [PubMed] [Google Scholar]
- 38.Bernstein AM, Pan A, Rexrode KM, Stampfer M, Hu FB, Mozaffarian D, et al. Dietary protein sources and the risk of stroke in men and women. Stroke 2012;43:637-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauvaget C, Nagano J, Allen N, Grant EJ, Beral V. Intake of animal products and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Int J Epidemiol 2003;32:536-43. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura Y, Iso H, Kita Y, Ueshima H, Okada K, Konishi M, et al. Egg consumption, serum total cholesterol concentrations and coronary heart disease incidence: Japan Public Health Center-based prospective study. Br J Nutr 2006;96:921-8. [DOI] [PubMed] [Google Scholar]
- 41.Qureshi AI, Suri FK, Ahmed S, Nasar A, Divani AA, Kirmani JF. Regular egg consumption does not increase the risk of stroke and cardiovascular diseases. Med Sci Monit 2007;13:CR1-8. [PubMed] [Google Scholar]
- 42.Djousse L, Gaziano JM. Egg consumption in relation to cardiovascular disease and mortality: the Physicians’ Health Study. Am J Clin Nutr 2008;87:964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burke V, Zhao Y, Lee AH, Hunter E, Spargo RM, Gracey M, et al. Health-related behaviours as predictors of mortality and morbidity in Australian Aborigines. Prev Med 2007;44:135-42. [DOI] [PubMed] [Google Scholar]
- 44.Spady DK, Woollett LA, Dietschy JM. Regulation of plasma LDL-cholesterol levels by dietary cholesterol and fatty acids. Annu Rev Nutr 1993;13:355-81. [DOI] [PubMed] [Google Scholar]
- 45.Clarke R, Frost C, Collins R, Appleby P, Peto R. Dietary lipids and blood cholesterol: quantitative meta-analysis of metabolic ward studies. BMJ 1997;314:112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howell WH, McNamara DJ, Tosca MA, Smith BT, Gaines JA. Plasma lipid and lipoprotein responses to dietary fat and cholesterol: a meta-analysis. Am J Clin Nutr 1997;65:1747-64. [DOI] [PubMed] [Google Scholar]
- 47.Greene CM, Waters D, Clark RM, Contois JH, Fernandez ML. Plasma LDL and HDL characteristics and carotenoid content are positively influenced by egg consumption in an elderly population. Nutr Metab (Lond) 2006;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mutungi G, Waters D, Ratliff J, Puglisi M, Clark RM, Volek JS, et al. Eggs distinctly modulate plasma carotenoid and lipoprotein subclasses in adult men following a carbohydrate-restricted diet. J Nutr Biochem 2010;21:261-7. [DOI] [PubMed] [Google Scholar]
- 49.Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 2010;363:2102-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER 3rd, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005;294:2455-64. [DOI] [PubMed] [Google Scholar]
- 51.Rosenblum JL, Castro VM, Moore CE, Kaplan LM. Calcium and vitamin D supplementation is associated with decreased abdominal visceral adipose tissue in overweight and obese adults. Am J Clin Nutr 2012;95:101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brandenburg VM, Vervloet MG, Marx N. The role of vitamin D in cardiovascular disease: from present evidence to future perspectives. Atherosclerosis 2012;225:253-63. [DOI] [PubMed] [Google Scholar]
- 53.Djousse L, Gaziano JM. Egg consumption and risk of heart failure in the Physicians’ Health Study. Circulation 2008;117:512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spence JD, Jenkins DJ, Davignon J. Egg yolk consumption and carotid plaque. Atherosclerosis 2012;224:469-73. [DOI] [PubMed] [Google Scholar]
- 55.Zampelas A. Still questioning the association between egg consumption and the risk of cardiovascular diseases. Atherosclerosis 2012;224:318-9. [DOI] [PubMed] [Google Scholar]
- 56.Fielding CJ, Castro GR, Donner C, Fielding PE, Reaven GM. Distribution of apolipoprotein E in the plasma of insulin-dependent and noninsulin-dependent diabetics and its relation to cholesterol net transport. J Lipid Res 1986;27:1052-61. [PubMed] [Google Scholar]
- 57.Venkatesan S, Imrie H, Read S, Halliday D. Apo C subclasses from non-insulin-dependent diabetic patients—a quantitative comparison with control subjects. Biochem Soc Trans 1995;23:278S. [DOI] [PubMed] [Google Scholar]
- 58.Howard BV. Insulin resistance and lipid metabolism. Am J Cardiol 1999;84:28-32J. [DOI] [PubMed] [Google Scholar]
- 59.Borggreve SE, De Vries R, Dullaart RP. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur J Clin Invest 2003;33:1051-69. [DOI] [PubMed] [Google Scholar]
- 60.Riemens SC, Van Tol A, Stulp BK, Dullaart RP. Influence of insulin sensitivity and the TaqIB cholesteryl ester transfer protein gene polymorphism on plasma lecithin:cholesterol acyltransferase and lipid transfer protein activities and their response to hyperinsulinemia in non-diabetic men. J Lipid Res 1999;40:1467-74. [PubMed] [Google Scholar]
- 61.Tanaka H, Ueda Y, Hayashi M, Date C, Baba T, Yamashita H, et al. Risk factors for cerebral hemorrhage and cerebral infarction in a Japanese rural community. Stroke 1982;13:62-73. [DOI] [PubMed] [Google Scholar]
- 62.Iso H, Jacobs DR Jr, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med 1989;320:904-10. [DOI] [PubMed] [Google Scholar]
- 63.Yano K, Reed DM, MacLean CJ. Serum cholesterol and hemorrhagic stroke in the Honolulu Heart Program. Stroke 1989;20:1460-5. [DOI] [PubMed] [Google Scholar]
- 64.Neaton JD, Blackburn H, Jacobs D, Kuller L, Lee DJ, Sherwin R, et al. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med 1992;152:1490-500. [PubMed] [Google Scholar]
- 65.Blood pressure, cholesterol, and stroke in eastern Asia. Eastern Stroke and Coronary Heart Disease Collaborative Research Group. Lancet 1998;352:1801-7. [PubMed] [Google Scholar]
- 66.Ooneda G, Yoshida Y, Suzuki K, Shinkai H, Hori S, Kobori K, et al. Smooth muscle cells in the development of plasmatic arterionecrosis, arteriosclerosis, and arterial contraction. Blood Vessels 1978;15:148-56. [DOI] [PubMed] [Google Scholar]
- 67.Tandon N, Harmon JT, Rodbard D, Jamieson GA. Thrombin receptors define responsiveness of cholesterol-modified platelets. J Biol Chem 1983;258:11840-5. [PubMed] [Google Scholar]
- 68.Westerterp KR, Goris AH. Validity of the assessment of dietary intake: problems of misreporting. Curr Opin Clin Nutr Metab Care 2002;5:489-93. [DOI] [PubMed] [Google Scholar]
- 69.Bingham SA, Luben R, Welch A, Wareham N, Khaw KT, Day N. Are imprecise methods obscuring a relation between fat and breast cancer? Lancet 2003;362:212-4. [DOI] [PubMed] [Google Scholar]
- 70.Kipnis V, Freedman LS. Impact of exposure measurement error in nutritional epidemiology. J Natl Cancer Inst 2008;100:1658-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Web tables