Figure 2. Kullback-Leibler Divergences show position-specific effects of lysine acetylation in HMGCS2.
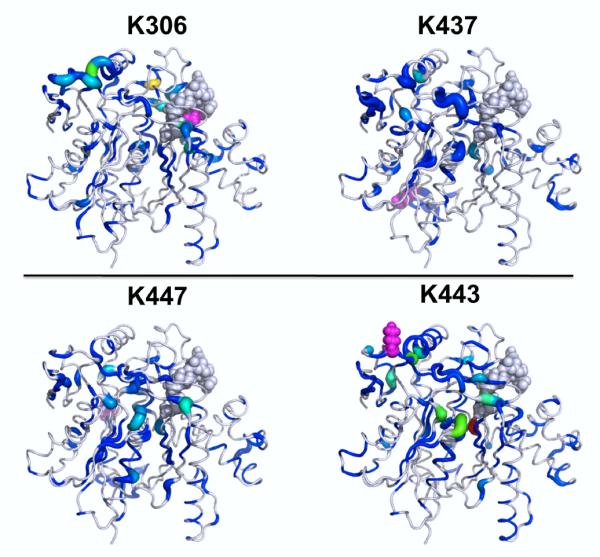
Local Kullback-Leibler Divergences between deacetylated and acetylated HMGCS2 conformational ensembles are given for different lysine acetylations (all are on the same scale). The lysine acetylated in each case is shown in purple spheres. (Top) Acetylation of Lys306 or Lys437 does not yield significant changes in structure and dynamics near the acetyl-CoA binding site (gray spheres) as assessed by the local Kullback-Leibler Divergence. (Bottom) In contrast to these negative controls, acetylation at lysine 447 or 443 causes substantial divergences proximal to the active site and the tail of the acetyl-CoA, and some background of divergences across the whole protein.
