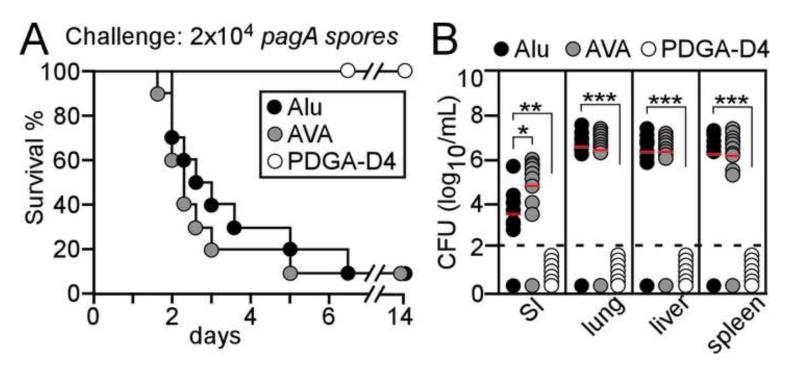
Fig. 6.
PDGA-D4 immunized guinea pigs are protected against anthrax challenge with pagA mutant spores. (A) Guinea pigs (n=10) were immunized with a prime-two booster schedule with either PDGA-D4 adsorbed to Alhydrogel or anthrax vaccine adsorbed (AVA / BioThrax®) or with adjuvant alone (Alu). Animals were challenged by subcutaneous inoculation with 2×104 spores derived from the B. anthracis Ames pagA variant and monitored over 14 days for disease and survival. (B) B. anthracis pagA replication in guinea pigs infected as described in (A) at either the site of infection (SI) or in lung, liver and spleen tissues. Bacterial load was determined by plating homogenized tissues on agar and enumerating colony formation. The Log-rank test was used to determine significance in animal survival (A): PDGA-D4 vs. Alu, P<0.001; PDGA-D4 vs. AVA, P<0.001. The two-tailed unpaired student’s t-test was used to analyze differences in B. anthracis load in guinea pig tissues of vaccine cohorts compared with adjuvant control (Alu): *P≤0.01, *P≤0.001, *P≤0.001. All animal data are representative of two independent determinations.