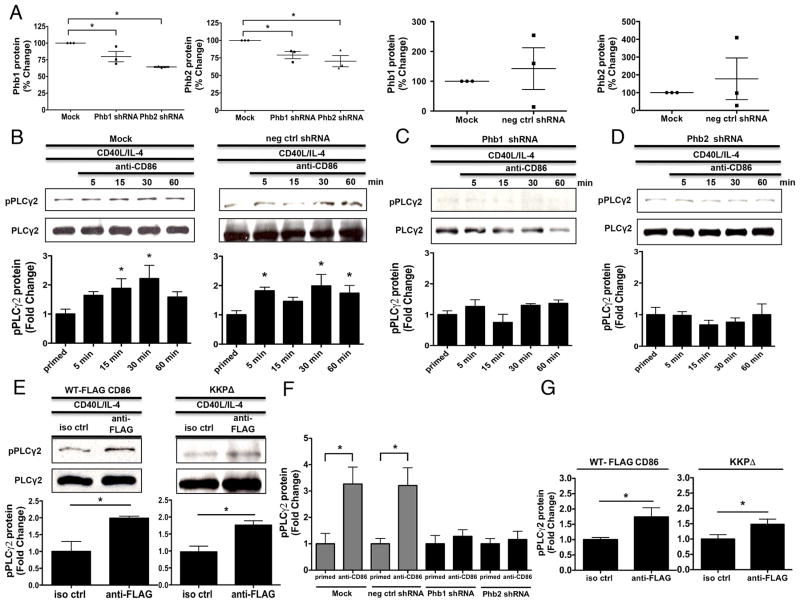
FIGURE 5.
Phb1 and Phb2 are necessary for the CD86-induced activation of PLCγ2. A, CH12.LX B cells were either Mock transfected, with scrambled negative control, or Phb1/2-specific shRNA plasmids via nucleofection for 24 hours followed by priming with CD40L/IL-4 for 16 hours. Phb1/2 protein levels were measured via immunoblot relative to GAPDH. Densitometry was performed and the data are expressed as the mean % Change + SEM relative to Mock and are representative of three independent experiments. B, CH12.LX B cells were either Mock transfected or with scrambled negative control shRNA (neg ctrl shRNA) via nucleofection for 24 hours followed by priming with CD40L/IL-4 for 16 hours. A CD86 Ab (anti-CD86) was administered for 5, 15, 30, and 60 minutes. Levels of phospho-PLCγ2 (pPLCγ2) protein relative to total PLCγ2 were measured via immunoblot. C and D, Phb1 or Phb2 shRNA plasmids were transfected into CH12.LX B cells followed by CD40L/IL-4 priming, and a CD86 Ab (anti-CD86) was added as described above. Levels of pPLCγ2 protein were measured relative to total PLCγ2 via immunoblot. E, WT, and CD86 cytoplasmic-deficient (KKPΔ) FLAG-CD86 plasmids were transfected into CH12.LX B cells via nucleofection and primed with CD40L/IL-4 for 16 hours. Either an anti-FLAG Ab, or species- and isotype-matched control Ab (iso ctrl Ab) was added for 15 minutes. Levels of pPLCγ2 protein were measured relative to total PLCγ2 via immunoblot. Representative gels (B to E) are shown from three independent experiments. Densitometry was performed and measured pPLCγ2 relative to PLCγ2 band intensity and the data are presented as the mean Fold Change in pPLCγ2 from primed B cells where CD86 was engaged relative to priming alone (B to D) or iso ctrl Ab (E) and expressed as the mean Fold Change ± SEM from three independent experiments. Statistical differences are shown relative to Mock (A), priming alone (B to D) or iso ctrl Ab (E). *, p < 0.05. F, CH12.LX B cells were either Mock transfected or with scrambled negative control shRNA (neg ctrl shRNA) via nucleofection for 24 hours followed by priming with CD40L/IL-4 for 16 hours. A CD86 Ab (anti-CD86) was administered for 30 minutes. Levels of phospho-PLCγ2 (pPLCγ2) protein relative to total PLCγ2 were measured via In-Cell ELISA. G, WT, and CD86 cytoplasmic-deficient (KKPΔ) FLAG-CD86 plasmids were transfected into CH12.LX B cells via nucleofection and primed with CD40L/IL-4 for 16 hours. Either an anti-FLAG Ab, or species-and isotype-matched control Ab (iso ctrl Ab) was added for 15 minutes. Levels of pPLCγ2 protein were measured relative to total PLCγ2 via In-Cell ELISA. (F & G) pPLCγ2 protein OD values were normalized to total pPLCγ2 protein OD values and the data are represented as a mean Fold Change in pPLCγ2 protein from CD86-engaged B cells relative to primed alone B cells of each group (F) or iso ctrl (G) and are expressed as the mean ± SD of quintuplicate samples/condition from at least two independent experiments. Statistical differences are shown relative to priming alone of each group (F) or iso ctrl (G). *, p < 0.05.