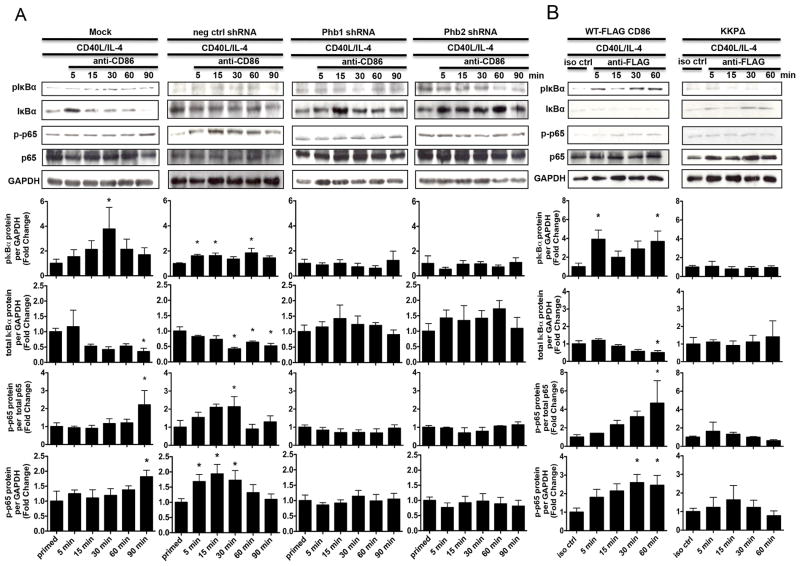
FIGURE 7.
Phb1/2 and the CD86 cytoplasmic domain are each required for the CD86- dependent activation of NF-κB. A, CH12.LX B cells were Mock transfected, transfected with either scrambled negative control shRNA, or with Phb1, or Phb2-specific shRNA plasmids via nucleofection for 24 hours followed by priming with CD40L/IL-4 for 16 hours. The cells were then resuspended in serum-free conditions for at least 30 minutes. A CD86 Ab (anti-CD86) was added to cell cultures for 5, 15, 30, 60, and 90 minutes. Levels of phospho-IκBα (pIκBα), total IκBα, and phospho-p65 (p-p65) protein were measured relative to GAPDH or total p65 via immunoblot. B, WT, or CD86-cytoplasmic deficient (KKPΔ) FLAG-CD86 plasmids were transfected into CH12.LX B cells via nucleofection and primed with CD40L/IL-4 for 16 hours. An anti-FLAG Ab was added for 5, 15, 30, and 60 minutes relative to a species- and isotype- matched control Ab (iso ctrl Ab). Levels of pIκBα, total IκBα, and p-p65 protein were measured relative to GAPDH or total p65 via immunoblot. Representative gels are shown from three independent experiments. Densitometry was performed and measured pIκBα, total IκBα, and p-p65 band intensity relative to GAPDH, and p-p65 band density relative to total p65 and the data are presented as the mean Fold Change in pIκBα, total IκBα, or p-p65 from primed B cells where CD86 was engaged relative to priming alone (A) or iso ctrl Ab (B) and expressed as the mean Fold Change ± SEM from at least three independent experiments. Statistical differences are shown relative to priming alone (A) or iso ctrl Ab (B). *, p < 0.05.