Abstract
Microbial keratitis is a sight-threatening complication associated with contact lenses. The introduction of silicone hydrogel lens materials with increased oxygen transmission to the ocular surface has not significantly altered the incidence of microbial keratitis. These data suggest that alternate, or additional, predisposing factors involving lens wear must be addressed to reduce or eliminate these infections. The contact lens can provide a surface for microbial growth in situ, and can also influence ocular surface homeostasis through effects on the tear fluid and corneal epithelium. Thus, it is intuitive that future contact lens materials could make a significant contribution to preventing microbial keratitis. Design of the “right” material to prevent microbial keratitis requires understanding the effects of current materials on bacterial virulence in the cornea, and on ocular surface innate defenses. Current knowledge in each of these areas will be presented, with a discussion of future directions needed to understand the influence of lens material on the pathogenesis of microbial keratitis.
Keywords: Microbial keratitis, contact lenses, corneal epithelium, Pseudomonas aeruginosa, innate defense, tear fluid, lens materials
INTRODUCTION
Microbial keratitis is an acute, and potentially serious complication of contact lens wear. Increasing resistance of infecting bacteria to antimicrobial therapy complicates the treatment of microbial keratitis. Even with successful therapy, visual outcomes of microbial keratitis often involve reduced visual acuity. Although microbial keratitis is a rare complication of contact lens wear, the number of lens wearers, the severity of infection, and the risk to vision provide important reasons to study this disease and ways to prevent it. Unfortunately, several decades of research have not yet solved the pathogenesis of microbial keratitis, but they have yielded many advances in the field that shed light on the details of infection and corneal susceptibility in human populations and animal models of infection. 1–3
It has become very clear that microbial keratitis is a multifactorial disease. While risk factors include overnight or extended-wear of contact lenses, poor patient compliance with lens care, and microbial contamination of lenses and cases, no one factor has yet been identified that determines infection susceptibility. Disappointingly, the advent of silicone hydrogel lenses, with significantly greater oxygen diffusion than conventional hydrogels, has not reduced the risk of microbial keratitis 4, 5. In a sense, however, this is perhaps not surprising. In every patient, the contact lens is being introduced for hours or days at a time into a complex dynamic system involving the tear film, the ocular surface, and the environment, with a constant exposure to microbes and their antigens. That system normally protects the eye from infection, but we do not fully understand how this occurs, nor do we know how the contact lens affects that dynamic. Understanding both will be needed for us to determine the critical combination of factors needed to allow infection.
The question to address in this article is; could contact lens material affect the pathogenesis of microbial keratitis? The immediate answer is that we don’t yet know, but it is clear that the contact lens could play a pivotal role in both bacterial virulence in the cornea and modulating corneal defenses against infection, and that future lens materials could make an important difference in solving this serious complication. This article will highlight some key mechanisms by which the contact lens could participate in microbial keratitis, and how future lens material alterations could reduce the significance of those events, and reduce or eliminate this disease.
LENS MATERIAL AND BACTERIAL COLONIZATION OF THE CONTACT LENS
Patients’ contact lenses, and especially lens cases, can have extraordinary levels and diversity of microbial contamination. 6 Although rates of lens/case contamination do not relate to the incidence of microbial keratitis, these microbes provide an obvious source of potentially causative pathogens when other prevailing conditions are suitable for infection. Lens materials provide a conduit for bacterial and fungal attachment that is influenced by lens material type, 7–9 with silicone hydrogel lenses actually showing greater adhesion of Pseudomonas aeruginosa and Staphylococcus aureus. 10, 11 As such, modification of current materials to prevent attachment or kill initially attaching microbes represents one mechanism for reducing the risk of infection. 12, 13 Lens-attached bacteria can readily form biofilms with enhanced resistance to antimicrobials, 14 and P. aeruginosa biofilms formed on contact lenses show greater density and viable bacteria when formed in the presence of phagocytic cells or corneal epithelial debris.15, 16 These data are consistent with our studies using a contact lens wear in vivo rodent model of Pseudomonas keratitis which showed extensive biofilm formation on posterior lens surfaces associated with the development of microbial keratitis 17. These infections occurred after several days of continuous lens wear suggesting that bacterial adaptation was needed to the ocular environment in order for infection to occur. That adaptation is likely to involve those biofilm-associated bacteria that could be protected from natural antimicrobial host defense factors present in the tears or secreted by the cornea. Ocular antimicrobial factors seem to be active since anterior lens surfaces showed minimal microbial colonization in the in vivo model. 17 Biofilms would not only offer a survival advantage, but also an opportunity for bacteria to adapt their gene expression to express phenotypes more suited to the prevailing ocular environment. Indeed, biofilms transferred from infected rodent eyes induced keratitis more rapidly than initially inoculated bacteria. 17 While this faster progression to disease may reflect transference of inflammatory mediators, or other in vivo factors, which compromise epithelial barrier function, it may also reflect the presence of adapted biofilm bacteria “primed” for infection of the cornea. Some of our other unpublished studies have also shown that P. aeruginosa adapts to traversal of human corneal epithelia with diverse changes in gene expression which have the potential to contribute to virulence. Interestingly, these traversed/adapted bacteria also formed extensive biofilm-like aggregates. Together these data suggest that contact lens material plays a key role in the pathogenesis of microbial keratitis in allowing bacterial adhesion and biofilm formation, and that modification of lens material to prevent attachment, inhibit bacterial viability or adaptive changes in gene expression, could have a tremendous impact in reducing the risk of infection. However, further studies are needed to determine which bacterial genes are critical for adhesion, adaptation and virulence on the posterior lens surface in vivo, and to understand whether biofilm or dispersed bacteria cause subsequent corneal infection. Results of those studies could then allow the design of contact lens material(s) that reduce or block these processes. Interestingly, using an in vivo rodent lens-wearing model of P. aeruginosa keratitis similar to that described above, silicone hydrogel lenses were associated with reduced risk of inflammation and infection compared to conventional hydrogel lenses suggesting that lens material can have an influence in vivo. 18 However, the relationship of all of these findings to infections in humans remains to be determined.
LENS MATERIAL AND CORNEAL DEFENSES AGAINST INFECTION
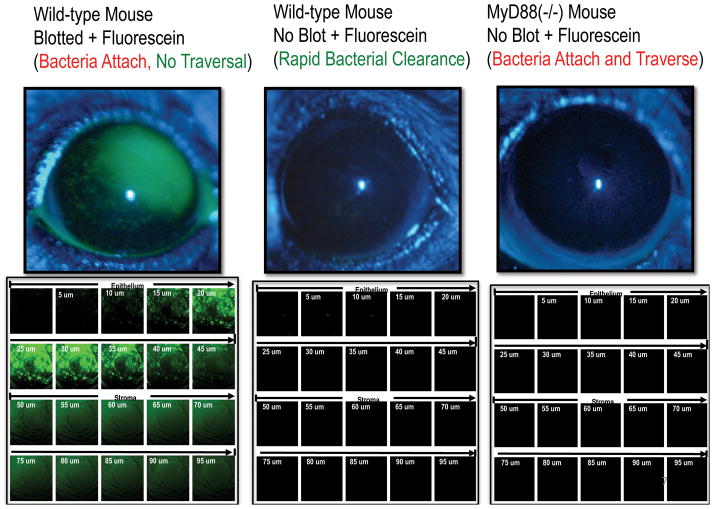
The contact lens is not only a potential conduit for bacterial attachment and adaptation in vivo, but can also influence the ocular surface environment and innate defenses against infection. One part of our studies of the pathogenesis of microbial keratitis is focused on normal defenses of the cornea against infection, and the mechanisms by which contact lenses may compromise those defenses. Indeed, the normal uninjured cornea is remarkably resistant to bacterial colonization and infection. In rodent models, we have shown that the introduction of large P. aeruginosa inocula into the uninjured eye, including cytotoxic clinical isolates that damage and kill corneal epithelial cells, results in a rapid clearance of bacteria within hours, and without tissue injury. 19 This “null-infection” model is proving very useful to study the pathogenesis of microbial keratitis, by allowing us to understand the normal innate defenses of the cornea, and how contact lenses may compromise these defenses to allow bacteria, such as P. aeruginosa, to adhere and traverse the corneal epithelium, the latter being a prerequisite for infection and disease. In recent studies, we have highlighted the importance of corneal innate defense in protection against P. aeruginosa. For example, using a null-infection in vivo model, we showed that normal mouse corneas are resistant to P. aeruginosa adherence and epithelial traversal, and show no fluorescein staining (Fig. 2, center panel). However, if the intact mouse cornea was blotted with tissue paper, similar to impression cytology, extensive fluorescein staining was observed (Fig. 2, left panel). Moreover, those corneas also allowed P. aeruginosa to bind to the corneal epithelium, but did not allow bacteria to traverse the epithelium or cause infection. 20, 21 Therefore, tissue paper blotting removed a key adhesion defense from the cornea, which allowed fluorescein staining, but did not allow infection. Fluorescein staining without subsequent infection was not surprising since we had previously observed a similar phenomenon in healing mouse corneas in vivo. 22 Interestingly, mice deficient in the innate defense adaptor protein MyD88, which controls the expression of numerous innate defense factors derived from toll-like receptor (TLR) or IL-1 receptor (IL-1R) mediated responses to bacteria, allowed P. aeruginosa to bind to the cornea, and readily traverse the corneal epithelium. The latter occurred without tissue paper blotting, and MyD88 knockout corneas showed no staining with fluorescein (Fig. 2, right panel) suggesting that their epithelial tight junctions were intact. Two-photon imaging of MyD88 knockout mouse corneas after inoculation with P. aeruginosa showed bacteria readily traversing the epithelium after 8 h (Fig. 3A), preceded by increased bacterial adherence to the seemingly intact corneal epithelium after 4 h (Fig. 3B). 21 These data show that MyD88-mediated corneal defenses, presumably to bacterial antigens, are critical for host defense against P. aeruginosa adhesion and epithelial traversal, but not through disruption of epithelial tight junctions, a previously known factor for increased corneal susceptibility to infection. These data also show that the cornea has independent defenses against P. aeruginosa adhesion and traversal when MyD88 is present.
Figure 2.
The “null-infection” murine model of corneal defenses against P. aeruginosa shows different defenses against adhesion and traversal, and that knockout of the MyD88 innate defense adaptor protein does not compromise epithelial tight junction integrity, while allowing P. aeruginosa to adhere and traverse the cornea (also see Figure 3). See references. 20, 21 (Modified from Tam et al. PLoS ONE. 2011; 6(8): e24008).
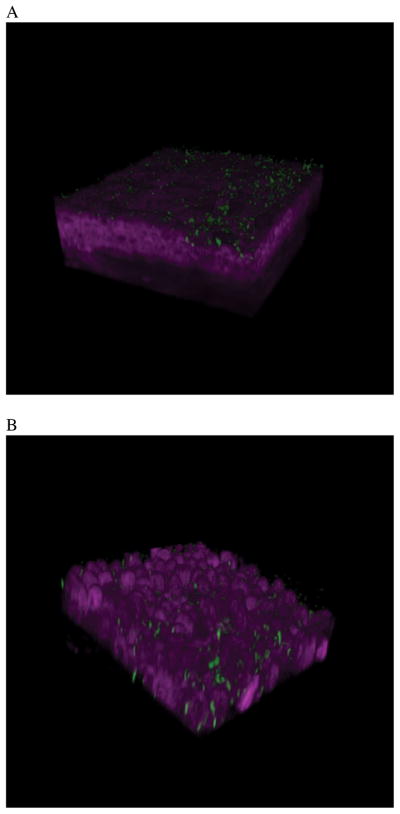
Figure 3.
Two-photon microscopy was used to show P. aeruginosa adhesion and then traversal of a MyD88 (−/−) mouse cornea at 4 h (A) and 8 h (B) post-inoculation. The cornea was not blotted with tissue paper prior to bacterial inoculation. However, bacteria readily attach after 4 h, and show extensive epithelial traversal after 8 h. A normal cornea does not show P. aeruginosa attachment or traversal. These data suggest that TLR and IL-1R signaling is significant for defending the cornea against microbial challenge. 21 (Modified from Tam et al. PLoS ONE. 2011; 6(8): e24008).
Cationic antimicrobial peptides represent one group of important corneal innate defense factors that are regulated by MyD88 either via TLR or IL-1R-mediated signaling. 23–25 We have shown that mouse beta defensin-3, the murine equivalent of human beta defensin 2, is involved in clearing P. aeruginosa from the ocular surface and preventing bacterial colonization. 26 Others have also shown that mBD-3 also participates in defense against P. aeruginosa at later stages of infection and disease. 27, 28 However, several years ago, we published a study showing that when contact lenses were placed on human corneal epithelial cells for 72 hours in vitro, the cells lost their ability to respond to bacterial antigens. 23 As a result, lens-exposed epithelial cells failed to up-regulate human beta defensin-2 (mRNA and protein) in response to P. aeruginosa antigens. Lens suppression of corneal epithelial innate defenses involved the suppression of JNK (c-Jun NH2-terminal MAP Kinase) and AP-1 activated stress response signaling in the epithelial cells, but did not affect expression of toll-like receptors (TLRs 2, 4, or 5). The mechanism for these effects is unknown, but the corneal epithelial cells appeared healthy, and lenses were extensively soaked in sterile saline to remove packaging solutions prior to cell exposure. These data still need to be confirmed in vivo, but suggest that lens material itself can influence corneal epithelial innate defenses. Interestingly, contact lenses did not suppress bacterial induced NFκB signaling in corneal epithelial cells, the latter transcription factor well known for its involvement in corneal pro-inflammatory cytokine secretion in response to bacterial antigens. 29, 30 Thus, lens material has potential to suppress certain pathways of innate defense, while allowing pro-inflammatory factor expression, an ideal situation for increasing epithelial vulnerability to bacterial attack. Nevertheless, further studies are needed to determine if/how lens material influences epithelial innate defense signaling in vitro and in vivo, and which changes in lens material design could help abrogate those effects.
Contact lens material also has the potential to influence other aspects of corneal epithelial defense beyond the suppression of antimicrobial factors. In vivo studies using lens-wearing rabbit models have shown contact lens wear can influence (decrease) epithelial cell proliferation in the central cornea, with the greatest effect observed for lenses with low oxygen transmissibility. 31 Similar models have also shown contact lens effects in slowing differentiation and renewal of the corneal epithelium in vivo. 32 Each of these lens-mediated effects could influence the ability of the corneal epithelium to form an effective barrier against P. aeruginosa and other pathogens. Indeed both hypoxia and contact lens wear have been associated with lipid raft formation and increased P. aeruginosa internalization into corneal epithelial cells in vitro and in vivo. 33, 34 Recent studies have also shown that lens materials and coatings can influence the cytotoxic effects of contact lens care solutions on corneal epithelial cells, e.g. cell health, and the expression of adhesion molecules. 35 It is not yet known how each of these lens-mediated effects influence the pathogenesis of microbial keratitis in humans, but it is clear that lens material effects on corneal epithelial cell biology (cell health, proliferation, differentiation, expression of adhesins, signaling) must be considered when designing lenses with a reduced risk of microbial keratitis.
LENS MATERIAL AND TEAR-MEDIATED DEFENSES
Tear fluid-mediated defenses against infection represent another element of ocular defense that could be influenced by lens material. Lens material could affect the volume of the post-lens tear film or the tear film composition; the latter through absorption/adsorption or inactivation of tear film components or compartmentalization of the tears, e.g. separation of proteases and protease inhibitors. Disruption of tear volume and/or composition could readily increase corneal susceptibility to infection. Our studies have shown that human tear fluid protects corneal epithelial cells from bacterial virulence mechanisms in vitro and virulence in vivo, through direct effects on bacterial cells (bacteriostatic/aggregation), and through an influence on corneal epithelial innate defenses. 36–38 In the latter instance, we have found that treatment of human corneal epithelial cells with human tear fluid protects the cells from P. aeruginosa invasion and cytotoxic effects through alteration of epithelial gene expression. Corneal genes for RNase7, an antimicrobial protein, and ST-2 an immune-modulator, were both significantly upregulated by human tear fluid with and without the presence of bacterial antigens. SiRNA knockdown showed that both genes helped protect against P. aeruginosa internalization. 38 Interestingly, these tear induced changes in corneal epithelial innate defense genes coincided with the up-regulation of both NFκB and AP-1 transcription factors suggesting that tears influence many other stress response and innate defense genes. Thus, not only could corneal innate defenses be potentially compromised by lens material through direct effects on the corneal epithelium, but also by lens-mediated effects on tear film volume and composition. It is well established that lens materials differ in their ability to adsorb tear film proteins and ocular mucins, 39–42 but it is not yet known how this impacts normal ocular innate defense against infection, or the pathogenesis of microbial keratitis.
CONCLUSION
So why does contact lens wear predispose the cornea to microbial keratitis, and could lens material affect disease pathogenesis? Although lens material has not yet made a difference in the incidence of microbial keratitis from a perspective of conventional hydrogels versus silicone hydrogels, it could readily do so in future. The lens is a conduit for microbial entry into the eye, attachment and biofilm formation, and other microbial adaptations that could provide microbes the time and opportunity for virulence, e.g. microbial traversal of the corneal epithelial barrier, and resistance to antimicrobial factors. The lens also disrupts the tear-film ocular surface interface, and the lens material can suppress corneal epithelial innate defense responses to microbial antigens in vitro resulting in reduced antimicrobial defenses against infection. Contact lenses can also interfere with normal epithelial proliferation and differentiation that may compromise barrier function. Lens interference with host defenses in vitro also takes time (several days) suggesting that, with current materials, lens effects on innate defenses (and microbial virulence) or are more likely to be prevalent with overnight or extended wear, known risk factors for infection. However, daily wear is also associated with microbial keratitis, and it would be of interest to determine if disease pathogenesis differs between these lens wear modalities. The bad news is that microbial keratitis seems to be a complex multifactorial disease involving microbial-host-lens interactions that are difficult to predict and model. Indeed, the dynamics of those interactions are likely to be further complicated by the potential for lens care solutions to affect any one, or more, of those components, i.e. the bacteria, corneal epithelium or lens material. The good news is that our knowledge today suggests that future lens materials could make a significant difference in reducing the risk of microbial keratitis by preventing microbial access and adaptation to the ocular surface, and preventing lens effects in compromising the corneal epithelium. It remains to be determined if silicone hydrogel materials will be part of that equation.
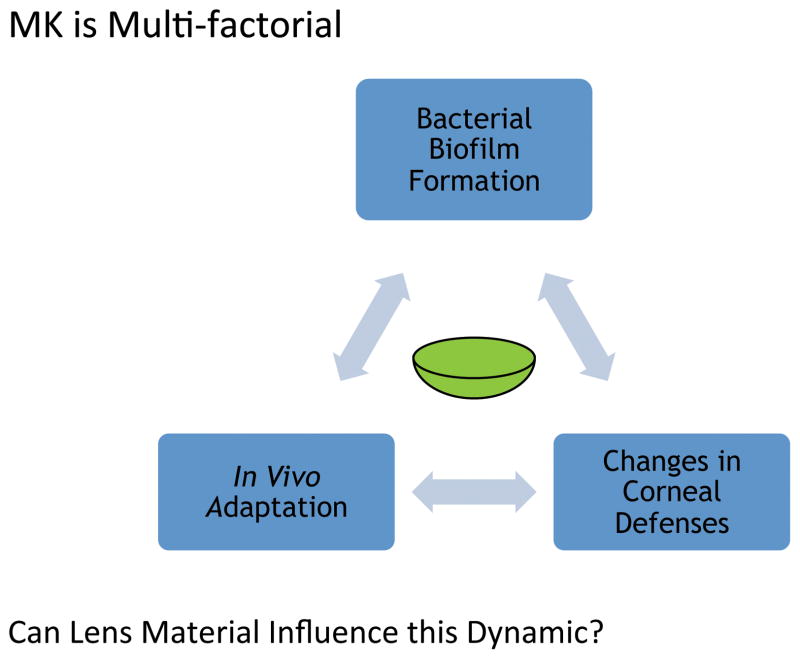
Figure 1.
The multifactorial nature of contact lens related microbial keratitis. The lens sits within the dynamic of corneal defense against infection (including tear-mediated defenses) and bacterial factors mediating infection and disease. The latter include lens colonization, biofilm formation and unknown in vivo adaptations that allow bacterial survival and virulence.
Acknowledgments
We are very grateful to our former and present students and colleagues at the Fleiszig-Evans Laboratory, School of Optometry, University of California, Berkeley for their hard work and commitment to understanding the pathogenesis of microbial keratitis. Special thanks to Drs. Danielle Augustin, James Mun, and Connie Tam for the use of their published and unpublished data included in this article. We are also very grateful to the National Eye Institute (EY011221), the National Institute of Allergy and Infectious Diseases (AI079192), and the Bill and Melinda Gates Foundation for research funding. Many thanks also to Alcon, Allergan, and CooperVision Inc. for their invaluable research support for our studies of P. aeruginosa keratitis.
Footnotes
Financial Disclosures: Dr. Fleiszig is a paid consultant for Allergan Inc. Drs. Evans and Fleiszig are named as co-inventors on several patents (held by University of California, Berkeley) related to use of ocular antimicrobial factors in preventing or treating corneal infections. These include defensins and collectins (e.g. surfactant proteins).
Approvals
All procedures involving animals were performed in strict accordance with a protocol approved by the Animal Care and Use Committee, University of California, Berkeley, an AAALAC accredited institution. Collection of tear fluid from human subject volunteers was performed using a protocol approved by the Committee for the Protection of Human Subjects, University of California, Berkeley.
References
- 1.Stapleton F, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond) 2012;26(2):185–93. doi: 10.1038/eye.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleiszig SM, Evans DJ. Pathogenesis of contact lens-associated microbial keratitis. Optom Vis Sci. 2010;87(4):225–32. doi: 10.1097/OPX.0b013e3181d408ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson DM, Cavanagh HD. The Clinical and Cellular Basis of Contact Lens-related Corneal Infections: A Review. Clin Ophthalmol. 2008;2(4):907–17. doi: 10.2147/opth.s3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dart JK, Radford CF, Minassian D, Verma S, Stapleton F. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008;115(10):1647–54. 54, e1–3. doi: 10.1016/j.ophtha.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Stapleton F, Keay L, Edwards K, Naduvilath T, Dart JK, Brian G, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115(10):1655–62. doi: 10.1016/j.ophtha.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Willcox MD, Carnt N, Diec J, Naduvilath T, Evans V, Stapleton F, et al. Contact lens case contamination during daily wear of silicone hydrogels. Optom Vis Sci. 2010;87(7):456–64. doi: 10.1097/OPX.0b013e3181e19eda. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Ahearn DG, Stulting RD, Schwam BL, Simmons RB, Pierce GE, et al. Differences among strains of the Fusarium oxysporum-F. solani complexes in their penetration of hydrogel contact lenses and subsequent susceptibility to multipurpose contact lens disinfection solutions. Cornea. 2007;26(10):1249–54. doi: 10.1097/ICO.0b013e318148bd9a. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher EL, Weissman BA, Efron N, Fleiszig SM, Curcio AJ, Brennan NA. The role of pili in the attachment of Pseudomonas aeruginosa to unworn hydrogel contact lenses. Curr Eye Res. 1993;12(12):1067–71. doi: 10.3109/02713689309033504. [DOI] [PubMed] [Google Scholar]
- 9.Fleiszig SM, Evans DJ, Mowrey-McKee MF, Payor R, Zaidi TS, Vallas V, et al. Factors affecting Staphylococcus epidermidis adhesion to contact lenses. Optom Vis Sci. 1996;73(9):590–4. doi: 10.1097/00006324-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kodjikian L, Casoli-Bergeron E, Malet F, Janin-Manificat H, Freney J, Burillon C, et al. Bacterial adhesion to conventional hydrogel and new silicone-hydrogel contact lens materials. Graefes Arch Clin Exp Ophthalmol. 2008;246(2):267–73. doi: 10.1007/s00417-007-0703-5. [DOI] [PubMed] [Google Scholar]
- 11.Willcox MD, Harmis N, Cowell, Williams T, Holden Bacterial interactions with contact lenses; effects of lens material, lens wear and microbial physiology. Biomaterials. 2001;22(24):3235–47. doi: 10.1016/s0142-9612(01)00161-2. [DOI] [PubMed] [Google Scholar]
- 12.Willcox MD. New strategies to prevent Pseudomonas keratitis. Eye Contact Lens. 2007;33(6 Pt 2):401–3. doi: 10.1097/ICL.0b013e318157e765. discussion 10–1. [DOI] [PubMed] [Google Scholar]
- 13.Selan L, Palma S, Scoarughi GL, Papa R, Veeh R, Di Clemente D, et al. Phosphorylcholine impairs susceptibility to biofilm formation of hydrogel contact lenses. Am J Ophthalmol. 2009;147(1):134–9. doi: 10.1016/j.ajo.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Szczotka-Flynn LB, Imamura Y, Chandra J, Yu C, Mukherjee PK, Pearlman E, et al. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea. 2009;28(8):918–26. doi: 10.1097/ICO.0b013e3181a81835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson DM, Parks QM, Young RL, Kret J, Poch KR, Malcolm KC, et al. Disruption of contact lens-associated Pseudomonas aeruginosa biofilms formed in the presence of neutrophils. Invest Ophthalmol Vis Sci. 2011;52(5):2844–50. doi: 10.1167/iovs.10-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnham GW, Cavanagh HD, Robertson DM. The impact of cellular debris on Pseudomonas aeruginosa adherence to silicone hydrogel contact lenses and contact lens storage cases. Eye Contact Lens. 2012;38(1):7–15. doi: 10.1097/ICL.0b013e31823bad0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam C, Mun JJ, Evans DJ, Fleiszig SM. The impact of inoculation parameters on the pathogenesis of contact lens-related infectious keratitis. Invest Ophthalmol Vis Sci. 2010;51(6):3100–6. doi: 10.1167/iovs.09-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Gabriel MM, Mowrey-McKee MF, Barrett RP, McClellan S, Hazlett LD. Rat silicone hydrogel contact lens model: effects of high- versus low-Dk lens wear. Eye Contact Lens. 2008;34(6):306–11. doi: 10.1097/ICL.0b013e3181891421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mun JJ, Tam C, Kowbel D, Hawgood S, Barnett MJ, Evans DJ, et al. Clearance of Pseudomonas aeruginosa from a healthy ocular surface involves surfactant protein D and is compromised by bacterial elastase in a murine null-infection model. Infect Immun. 2009;77(6):2392–8. doi: 10.1128/IAI.00173-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alarcon I, Tam C, Mun JJ, LeDue J, Evans DJ, Fleiszig SM. Factors impacting corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Invest Ophthalmol Vis Sci. 2011;52(3):1368–77. doi: 10.1167/iovs.10-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tam C, LeDue J, Mun JJ, Herzmark P, Robey EA, Evans DJ, et al. 3D quantitative imaging of unprocessed live tissue reveals epithelial defense against bacterial adhesion and subsequent traversal requires MyD88. PLoS One. 2011;6(8):e24008. doi: 10.1371/journal.pone.0024008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee EJ, Evans DJ, Fleiszig SM. Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Invest Ophthalmol Vis Sci. 2003;44(12):5220–7. doi: 10.1167/iovs.03-0229. [DOI] [PubMed] [Google Scholar]
- 23.Maltseva IA, Fleiszig SM, Evans DJ, Kerr S, Sidhu SS, McNamara NA, et al. Exposure of human corneal epithelial cells to contact lenses in vitro suppresses the upregulation of human beta-defensin-2 in response to antigens of Pseudomonas aeruginosa. Exp Eye Res. 2007;85(1):142–53. doi: 10.1016/j.exer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Redfern RL, Reins RY, McDermott AM. Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells. Exp Eye Res. 2011;92(3):209–20. doi: 10.1016/j.exer.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDermott AM. The role of antimicrobial peptides at the ocular surface. Ophthalmic Res. 2009;41(2):60–75. doi: 10.1159/000187622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Augustin DK, Heimer SR, Tam C, Li WY, Le Due JM, Evans DJ, et al. Role of defensins in corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Infect Immun. 2011;79(2):595–605. doi: 10.1128/IAI.00854-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu M, McClellan SA, Barrett RP, Zhang Y, Hazlett LD. Beta-defensins 2 and 3 together promote resistance to Pseudomonas aeruginosa keratitis. J Immunol. 2009;183(12):8054–60. doi: 10.4049/jimmunol.0902140. [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, McClellan SA, Barrett RP, Berger EA, Zhang Y, Hazlett LD. VIP and growth factors in the infected cornea. Invest Ophthalmol Vis Sci. 2011;52(9):6154–61. doi: 10.1167/iovs.10-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Wu XY, Yu FS. Inflammatory responses of corneal epithelial cells to Pseudomonas aeruginosa infection. Curr Eye Res. 2005;30(7):527–34. doi: 10.1080/02713680590968150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar A, Zhang J, Yu FS. Innate immune response of corneal epithelial cells to Staphylococcus aureus infection: role of peptidoglycan in stimulating proinflammatory cytokine secretion. Invest Ophthalmol Vis Sci. 2004;45(10):3513–22. doi: 10.1167/iovs.04-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladage PM, Yamamoto K, Ren DH, Li L, Jester JV, Petroll WM, et al. Proliferation rate of rabbit corneal epithelium during overnight rigid contact lens wear. Invest Ophthalmol Vis Sci. 2001;42(12):2804–12. [PubMed] [Google Scholar]
- 32.Ladage PM, Jester JV, Petroll WM, Bergmanson JP, Cavanagh HD. Vertical movement of epithelial basal cells toward the corneal surface during use of extended-wear contact lenses. Invest Ophthalmol Vis Sci. 2003;44(3):1056–63. doi: 10.1167/iovs.02-0725. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto N, Yamamoto N, Petroll MW, Cavanagh HD, Jester JV. Internalization of Pseudomonas aeruginosa is mediated by lipid rafts in contact lens-wearing rabbit and cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(4):1348–55. doi: 10.1167/iovs.04-0542. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto N, Yamamoto N, Jester JV, Petroll WM, Cavanagh HD. Prolonged hypoxia induces lipid raft formation and increases Pseudomonas internalization in vivo after contact lens wear and lid closure. Eye Contact Lens. 2006;32(3):114–20. doi: 10.1097/01.icl.0000177384.27778.4c. [DOI] [PubMed] [Google Scholar]
- 35.Gorbet MB, Tanti NC, Crockett B, Mansour L, Jones L. Effect of contact lens material on cytotoxicity potential of multipurpose solutions using human corneal epithelial cells. Mol Vis. 2011;17:3458–67. [PMC free article] [PubMed] [Google Scholar]
- 36.Fleiszig SM, Kwong MS, Evans DJ. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect Immun. 2003;71(7):3866–74. doi: 10.1128/IAI.71.7.3866-3874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong MS, Evans DJ, Ni M, Cowell BA, Fleiszig SM. Human tear fluid protects against Pseudomonas aeruginosa keratitis in a murine experimental model. Infect Immun. 2007;75(5):2325–32. doi: 10.1128/IAI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mun JJ, Tam C, Evans DJ, Fleiszig SM. Modulation of epithelial immunity by mucosal fluid. Sci Rep. 2011;1:8. doi: 10.1038/srep00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carney FP, Morris CA, Milthorpe B, Flanagan JL, Willcox MD. In vitro adsorption of tear proteins to hydroxyethyl methacrylate-based contact lens materials. Eye Contact Lens. 2009;35(6):320–8. doi: 10.1097/ICL.0b013e3181becd3c. [DOI] [PubMed] [Google Scholar]
- 40.Berry M, Harris A, Corfield AP. Patterns of mucin adherence to contact lenses. Invest Ophthalmol Vis Sci. 2003;44(2):567–72. doi: 10.1167/iovs.02-0720. [DOI] [PubMed] [Google Scholar]
- 41.Sack RA, Jones B, Antignani A, Libow R, Harvey H. Specificity and biological activity of the protein deposited on the hydrogel surface. Relationship of polymer structure to biofilm formation. Invest Ophthalmol Vis Sci. 1987;28(5):842–9. [PubMed] [Google Scholar]
- 42.Luensmann D, Glasier MA, Zhang F, Bantseev V, Simpson T, Jones L. Confocal microscopy and albumin penetration into contact lenses. Optom Vis Sci. 2007;84(9):839–47. doi: 10.1097/OPX.0b013e3181559ea4. [DOI] [PubMed] [Google Scholar]