Abstract
The adducin family of proteins associates with the actin cytoskeleton in a calcium-dependent manner. Beta adducin (βAdd) is involved in synaptic plasticity in the hippocampus; however, the role of βAdd in synaptic plasticity in other brain areas is unknown. Using diolistic labeling with the lipophilic dye DiI, we found that the density of mature mushroom-shaped spines was significantly decreased in the nucleus accumbens (NAc) in brain slices from βAdd KO mice as compared to their wildtype (WT) siblings. The effect of 10 days of daily cocaine (15 mg/kg) administration on NAc spine number and locomotor behavior was also measured in β Add WT and knockout (KO) mice. As expected, there was a significant increase in overall spine density in NAc slices from cocaine-treated WT mice at this time-point, however, there was a greater increase in the density of mushroom spines in β Add KO animals following chronic cocaine administration compared to WT. In addition, β Add KO mice showed elevated locomotor activity in response to cocaine treatment compared to WT siblings. These results indicate that β Add is required for stabilizing mature spines under basal conditions in the NAc, but that lack of this protein does not prevent synaptic remodeling following repeated cocaine administration. In addition, these data are consistent with previous studies suggesting that β Add may normally be involved in stabilizing spines once drug- or experience-dependent remodeling has occurred.
Keywords: dendritic spines, diolistic labeling, behavioral sensitization, synaptic plasticity, cocaine, adducin, medium spiny neurons, nucleus accumbens, DiI
Introduction
Several studies have shown that repeated administration of cocaine results in increases in spine density in the nucleus accumbens (NAc) (Pulipparacharuvil et al. 2008; Shen et al. 2009). Synaptic remodeling is known to require alterations of the cytoskeleton, but the molecular mechanisms underlying those alterations induced by cocaine are not well understood. Adducins are cytoskeleton-associated proteins that are phosphorylated in the NAc in response to cocaine administration (Toda et al, 2006; Lavaur et al, 2009). Adducins can cap and bundle actin filaments and can promote spectrin binding (Gardner and Bennett, 1987; Mische et al., 1987; Kuhlman et al., 1996). Calmodulin binding and phosphorylation by protein kinase A and protein kinase C cause adducin to dissociate from the actin cytoskeleton, allowing alterations in actin polymerization to occur (Matsuoka et al., 1996, 1998). Thus, adducins may contribute to activity-dependent cytoskeletal remodeling because they could provide a link between intracellular signaling pathways induced by neuronal activity and structural changes in the cytoskeleton.
Three genes (α, β and γ) encode mammalian adducin proteins and several splice variants have been identified (Suriyapperuma et al. 2000). Functional adducin is composed of α/β or α/γ heterodimers or heterotetramers (Dong et al. 1995; Hughes and Bennett 1995). Interestingly, α and γ adducin are expressed ubiquitously whereas the β subunit of adducin is enriched in brain and erythrocytes (Gilligan et al. 1999). Late-stage long-term potentiation is impaired in the CA1 field of the hippocampus in knockout (KO) mice lacking βadducin (β Add) despite relatively normal development of synaptic plasticity in the first hour after high-frequency stimulation (Rabenstein et al. 2005). This study suggests that adducin is important for the establishment of long-term morphological changes related to synaptic plasticity following early-stage biochemical changes.
In the current study, we analyzed spine density in the NAc of cocaine-naiveβAdd KO mice and their WT siblings. Spine morphology was examined using diolistic labeling with the lipophilic dye DiI followed by quantitation using Neuron Studio and IMARIS software. Changes in spine density in the NAc have been reported following chronic administration of a number of drugs of abuse including cocaine (Robinson and Kolb 1999; Robinson et al. 2001). Since the adducins are cytoskeleton-associated proteins that may be involved in alterations in actin dynamics underlying spine formation, we hypothesized that the ability of adducins to contribute to actin dynamics might suggest a role for β Add in cocaine-dependent spine remodeling, using a regimen that has been used previously to induce spine changes in the NAc of C57BL/6J mice (Lee et al. 2006). Finally, to determine whether the spine density changes in βAdd KO mice might be correlated with alterations in a behavior thought to relate to changes in synaptic strength in the NAc, we evaluated cocaine-induced locomotor sensitization in βAdd KO mice and their WT siblings. The data presented here show that β Add may be important for regulating mature dendritic spine density at baseline, but the protein is not required for spine remodeling in response to repeated cocaine administration.
Material and Methods
Animals
βAdd KO mice aged 8-12 months were generated as reported previously (Gilligan et al., 1999). The βAdd mouse line was backcrossed onto the C57BL/6J background (The Jackson Laboratory, Bar Harbor, ME) for at least 13 generations. Male littermate controls were used for all experiments. Mice were group-housed with no more than 5 animals per cage and kept in a colony room at 22°C on a 12 h light/dark cycle (lights on at 7:00 A.M.). Food and water were available ad libitum . All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Yale Animal Care and Use Committee.
Confocal imaging and quantitation of dendritic spines
Mice were administered saline (wildtype (WT): n = 11, KO: n = 18) or cocaine (15 mg/kg, i.p.; WT: n = 12, KO: n = 16) every day for 10 days, locomotion was measured as described below, and mice were then left undisturbed in their home cages for 2 weeks. Two weeks after the last cocaine injection, the same animals used for behavioral analysis were challenged with a single intraperitoneal (i.p.) administration of cocaine (15 mg/kg) and 15 min later, mice were perfused (5 ml/min) with 300 ml of 4% paraformaldehyde in phosphate buffered saline (PBS). Brains were removed and postfixed in the same fixative for 1 h, then were coronally sectioned (150 μm thick) using a vibratome at room temperature. Data were obtained from animals with the researcher blind to condition.
Diolistic labeling
Tungsten particles (1.3 μm diameter, Bio-Rad, Berkeley, CA) were coated with the lipophilic carbocyanine dye DiI (Invitrogen, Calrsbad, CA). DiI-coated particles were delivered diolistically into tissue at 120 psi using a Helios Gene Gun system (Bio-Rad, Berkeley, CA) fitted with a polycarbonate filter (3.0 μm pore size; BD Biosciences, Franklin Lakes, NJ). DiI was allowed to diffuse along neuronal dendrites and axons in PBS for 16 - 24 h at 4°C, and then labeled sections were fixed again in 4% paraformaldehyde for 1 h. After brief washing in PBS, sections were mounted with Fluoromount G mounting media (Electron Microscopy Sciences, Hatfield, PA) onto glass slides (Thermo Scientific, Waltham, MA).
Confocal imaging and three-dimensional reconstructions
Medium spiny neurons were imaged using an UltraVIEW VoX spinning disc confocal microscope with a 60x CFI Plan Apo objective and equipped with a Hamamatsu C9100-50 camera, with excitation at 543 nm. Z-stacks were collapsed into projection images and 2- 3 neurons/brain were manually analyzed per section.
Measurement of spine density and size
For initial quantitative analysis, collected data were processed for reconstruction with NeuronStudio software as described in the manufacturer's manual (CNIC, Mt. Sinai School of Medicine, New York, NY; http://research.mssm.edu/cnic/help/ns/index.html). The entire dendritic branch was analyzed, with lengths of 120-130 uM. Maximum and minimum spine height was set at < 3.5 um and > 0.5 um respectively. Minimum stubby spine size was set at > 22 voxels. After automatic detection of spines by the NeuronStudio software, spots that were not on the quantified dendrite were removed manually. Medium spiny neurons in nucleus accumbens were imaged across core and shell.
For alternative automated spine analysis, a three-dimensional perspective was rendered by the Surpass module of IMARIS software package (Version 5.5, Bitplane), as has been described in detail previously (Shen et al., 2008, 2009; Kroener et al., 2012). To increase the accuracy of identification of the surfaces of dendrite shaft and spines, images were deconvolved to reduce point-spread functions using the adaptive blind 3D deconvolution method (AutoQuant X, Version X2.1.3, Media Cybermedics, Inc., Bethesda, MD) prior to analysis. Using the Imaris XT (Bitplane AG, version 6.2.1, Zurich, Switzerland) module, a filament of the dendritic shaft and spines was created from ~50 μm length sections starting 30-50 μm from the soma, following previously reported methods (Shen et al., 2008, 2009; Kroener et al., 2012). Dendritic spines were then classified into 4 categories (i.e., mushroom, thin, stubby) based on their neck and head morphology, where L is spine length, WH is spine head width, and WN is spine neck width. Following these criteria, long spines were identified as having a mean WH ≥ mean WN, mushroom spines were identified as having a L < 3 and maximum WH > mean WN * 2, and stubby spines were identified as having a L < 0.5 μm. The remaining spines were identified as filopodia, and were typically observed to be long, thin protuberances stemming from the dendritic shaft.
Locomotor activity measurements
Mice (WT treated with saline: n=11, WT treated with cocaine: n=18, KO treated with saline: n=12, KO treated with cocaine: n=16) were placed in 45 × 24 × 20 cm clear plastic cages between two rows of photocells (8 total, 4 cm apart) for 30 min. This time interval for measuring locomotor activity was chosen because previous studies have shown that locomotor activity plateaus after 30 min. Locomotor activity was assessed as beam breaks per min using personalized Windows-compatible software. Beam breaks were compiled into four 5 min bins for analysis. Statistical analysis was performed using ANOVA with Tukey's-HSD post-hoc tests when relevant.
Data analysis
The spine data were statistically analyzed using a one- or two-way ANOVA. For comparing spine density the data were calculated as percentage change from saline values and compared using a two-tailed Student's t test. Post hoc tests were conducted using Tukey's-HSD test for repeated measures ANOVA
Results
Naïve βAdd knockout mice have a lower density of dendritic spines in the NAc
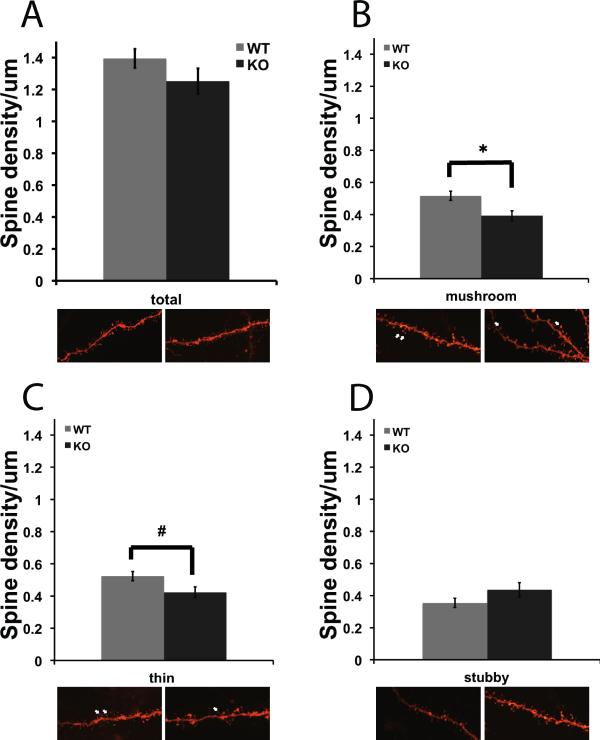
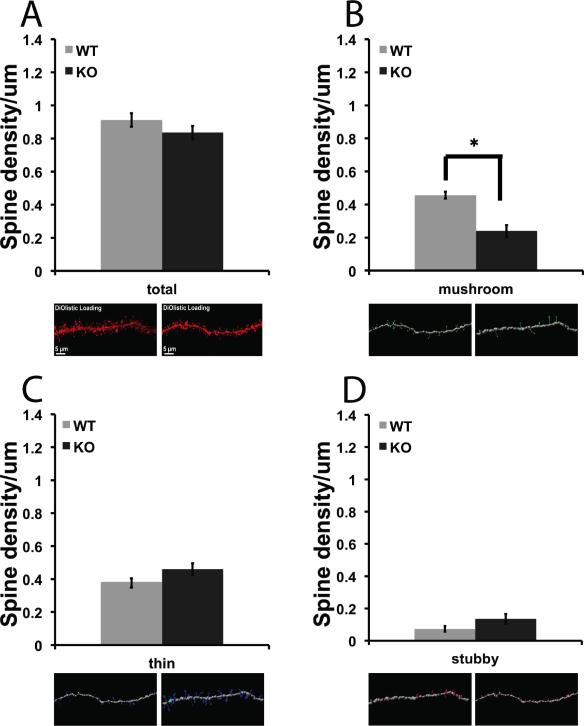
Dendritic spines on medium spiny neurons in the NAc were visualized with the lipophilic dye DiI and spine density was measured using NeuronStudio software for 3-D image analysis. Spines were measured, and classified as thin, mushroom or stubby subtypes. There was a significant difference in the number of mushroom and thin spine subtypes on NAc neurons from βAdd WT and KO mice (Fig. 1. B; F1, 47 = 8.723, p = 0.005, Fig. 1. C; F1, 47 = 5.116, p = 0.028), with a significant decrease in the density of both mushroom and thin spines at baseline in βAdd KO mice as compared to WT mice.
Figure 1. βAdd KO mice show decreased mushroom spine density at baseline.
Dendritic spines on medium spiny neurons in the NAc were labeled with DiI and analyzed using NeuronStudio software. Spines were classified as A) thin, B) mushroom or C) stubby. There was a significant decrease in the number of mushroom spines (B; * p ≤ 0.05, post hoc analysis, Tukey's-HSD). There was a significant decrease in density of thin spines in the NAc of βAdd KO mice as compared to WT mice (n=28 neurons from 11 WT saline treated mice, 43 neurons from 12 WT cocaine treated mice, 21 neurons from 18 KO saline treated mice, 29 neurons from 16 cocaine treated KO mice; p ≤ 0.05, post hoc analysis, Tukey's-HSD).
Constitutive knockout of βAdd does not prevent spine remodeling in response to cocaine treatment
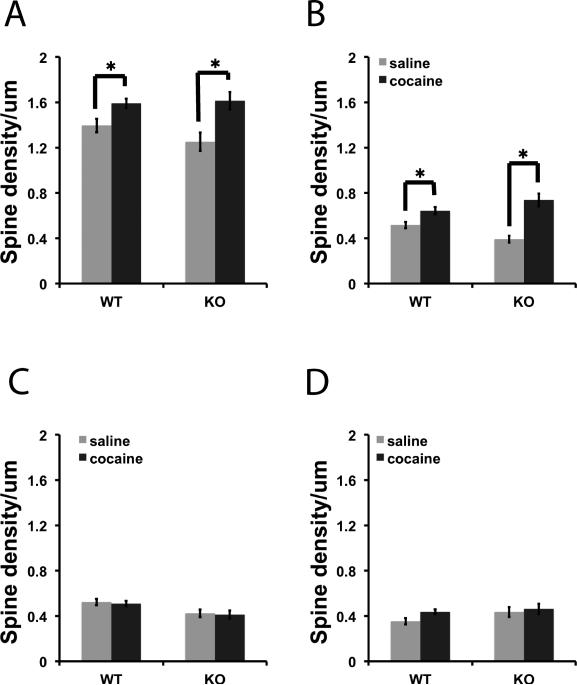
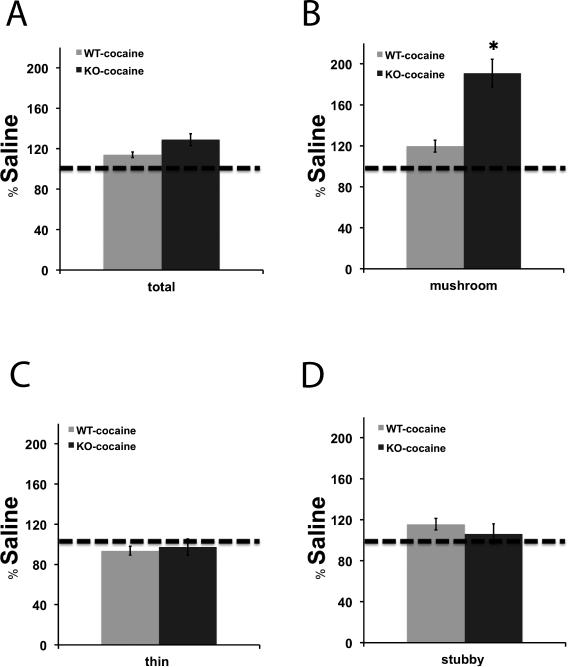
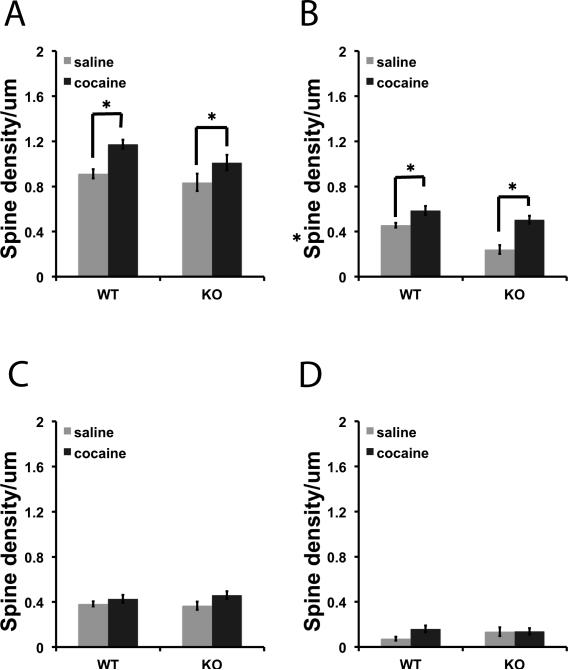
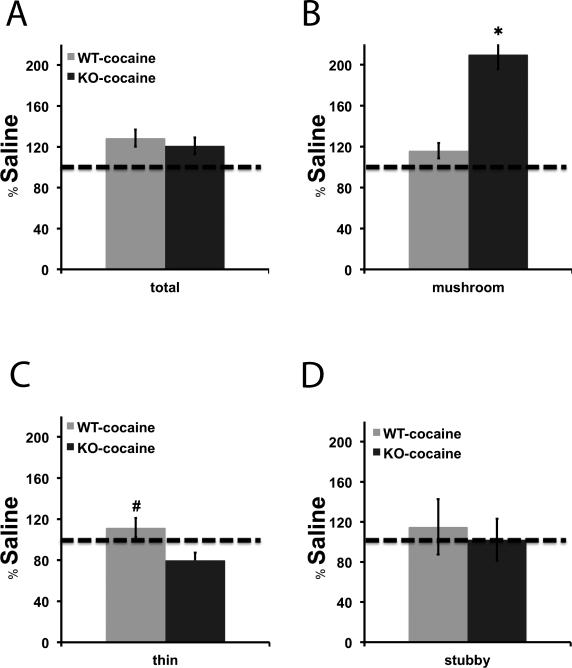
We measured spine density in the NAc of βAdd KO mice and their WT siblings following a single challenge dose of cocaine (15 mg/kg, i.p.) 2 weeks after repeated cocaine administration (15 mg/kg, i.p.; 10 days). Dendritic spines were classified as thin, mushroom, and stubby spines based on the ratio of head and neck diameter. Spine density (spine number/μm) was used as the primary outcome measure for analysis (Fig. 2) and was also measured as percentage change from basal spine density (Fig. 3). Cocaine treatment resulted in a significant increase in the density of spines in both WT and KO mice (Fig. 2A; F1, 121 = 6.875, p < 0.01, Fig. 2B; F1, 121 = 12.624, p < 0.001). Despite the similar increase in overall spine density, there was a much greater percent increase in mushroom spines in the NAc following cocaine treatment in βAdd KO mice as compared to neurons from WT βAdd mice (Fig. 3B; t31 = 4.795, p = 1.4 × 10-5).
Figure 2. Cocaine treatment significantly increases overall spine density in βAdd KO and WT mice.
Following repeated cocaine administration (15 mg/kg, , i.p.; 10 days), spine density was measured in the NAc of βAdd KO mice and their WT siblings. Density (spine number/um) of A) Total, B) mushroom, C) thin, and D) stubby spines was measured using NeuronStudio. There was a significant increase in the number of mushroom and thin spines in the NAc of βAdd WT and KO mice following cocaine treatment (*, p ≤ 0.05 post hoc analysis, Tukey's-HSD).
Figure 3. Mushroom spine density is greatly increased in βAdd KO mice following cocaine treatment.
The percentage change in density of A) total, B) mushroom, C) thin, and D) stubby spines over saline-treatment was measured following repeated cocaine administration (15 mg/kg, , i.p.; 10 days), spine density was measured in the NAc of βAdd KO mice and their WT siblings using NeuronStudio. There was a significant increase in the density of mushroom spines in the NAc of βAdd KO mice following cocaine treatment (*, p ≤ 0.05).
Similar pattern of spine density changes observed with an automated analysis procedure
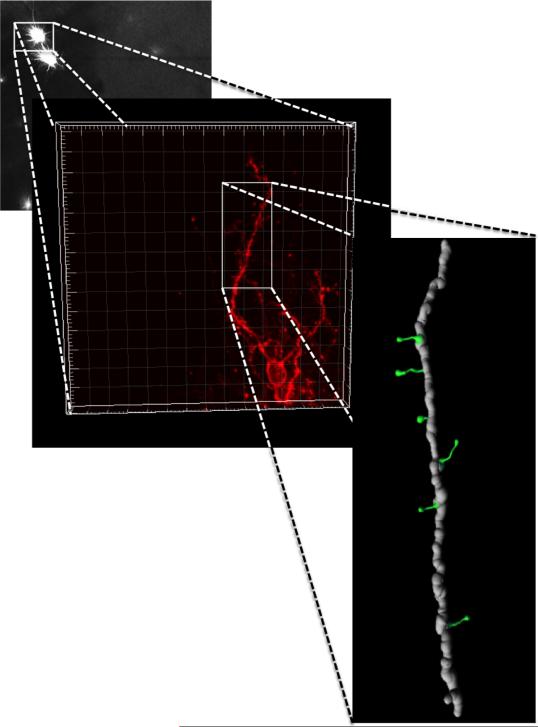
Dendritic spines can be classified into individual subtypes including stubby, mushroom, thin and branched, but each software program uses different spine detection algorithms. We therefore used an independent method to analyze spine density and spine morphology to verify the findings obtained using NeuronStudio. As a result, we re-analyzed the images obtained as described above using IMARIS software with 3-dimensional image reconstruction (Fig. 4). The IMARIS software classifies spines into filopodia, thin, mushroom, and stubby spines. The IMARIS software had more stringent requirements for images that could be analyzed, so some images were excluded and the “n” size for these measurements was therefore somewhat lower than the analysis using NeuronStudio. Consistent with the findings obtained using NeuronStudio, there was a significant interaction between genotype and spine number, and a significant decrease in mushroom spines at baseline in the NAc of βAdd KO mice as compared to WT mice (Fig. 5B: F1,11 = 26.087, p=0.001, Fig. 5C; F1,11 = 3.754, p=0.085). Cocaine treatment also similarly resulted in a significant increase in spine density in both WT and βAdd KO mice (Fig. 6A; F1, 32 = 3.603, p = 0.024, Fig. 6B; F1, 32 = 10.956, p < 0.01). In addition, repeated cocaine administration significantly increased the number of mushroom spines in the NAc of βAdd KO mice (Fig. 7B; t10 = 4.933, p=0.001, Fig. 7C; t10 = 1.188, p=0.088).
Figure 4. Three-dimensional reconstruction of confocal images.
DiI labeled medium spiny neurons in NAc were imaged and DiI was excited using the Helium/Neon 543 nm laser line. Each neuron was scanned at 0.15μm intervals along the z-axis (maximum 90 planes, depending on the depth of whole neuron and objective WD), and the morphology of the dendritic tree was reconstructed in 3-D for analysis with IMARIS software.
Figure 5. Analysis using IMARIS software confirms a significant baseline decrease in NAc spine number in βAdd KO mice.
Using IMARIS software as an independent method of spine analysis, the density (spine number/μm) of A) total, B) mushroom, C) thin, and D) stubby spines was measured. There was a decrease in mushroom spine number in βAdd KO mice as compared to their WT siblings at baseline (*, p ≤ 0.05 post hoc analysis, Tukey's-HSD).
Figure 6. Cocaine treatment significantly increases spine density in βAdd KO and WT mice as analyzed using IMARIS software.
Following repeated cocaine administration (15 mg/kg, , i.p.; 10 days), spine density was measured in the NAc of βAdd KO mice and their WT siblings using IMARIS software. Density (spine number/um) of A) Total, B) mushroom, C) thin, and D) stubby spines was measured. There was a significant increase in the number of mushroom and thin spines in the NAc of βAdd WT and KO mice following cocaine treatment (*, p ≤ 0.05 for post hoc comparison between cocaine treatment and saline treatment in WT and KO mice, Tukey's-HSD).
Figure 7. An independent analysis method confirms that mushroom spine density is selectively increased in βAdd KO mice.
The percentage change in density of A) total, B) mushroom, C) thin, and D) stubby spines over saline-treatment was measured following repeated cocaine administration (15 mg/kg, , i.p.; 10 days), spine density was measured in the NAc of βAdd KO mice and their WT siblings using IMARIS software. There was a significant increase in the density of mushroom spines in the NAc of βAdd KO mice (*, p ≤ 0.05).
Enhanced locomotor sensitization in βAdd KO mice
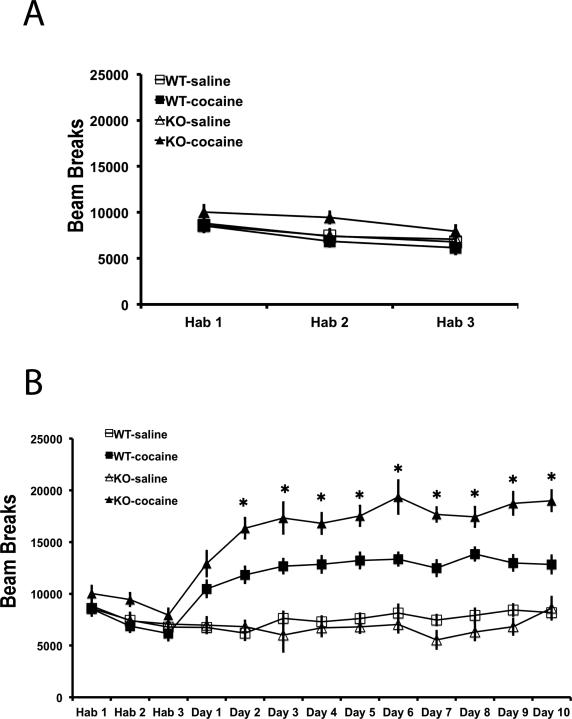
We evaluated cocaine-induced locomotor sensitization in βAdd KO mice and their WT siblings to determine whether spine density changes might be correlated behavior. Mice were treated with saline for three days to habituate the mice to injections and to the novel environment. There were no significant differences in locomotor activity between βAdd WT and βAdd KO mice over the three days of habituation (Fig. 8A). Following habituation, cocaine (15 mg/kg, i.p.) was administered once a day for 10 days, and locomotor activity was monitored for 30 min after cocaine administration. Both WT and βAdd KO mice showed significantly enhanced locomotor activity following acute cocaine administration (Fig. 8B). In addition, both groups of animals showed a sensitized locomotor response after 10 days of cocaine administration. Analysis of locomotor activity over daily sessions identified a significant interaction between genotype and treatment (Fig. 8B; Treatment × Genotype, F1,34 = 7.691, p=0.009). βAdd KO mice showed enhanced locomotor activity compared to WT mice across all 9 days of testing (Fig. 8B; * p ≤ 0.05 for post hoc comparison between and cocaine-treated WT and KO mice, Tukey's-HSD).
Figure 8. βAdd KO mice show an increase in cocaine-induced locomotor activity.
βAdd WT and KO mice were A) habituated to the locomotor apparatus for 3 days and then B) administered cocaine (15 mg/kg, i.p.) once a day for 10 days (WT-Sal: n=11, WT-Coc: n=18, KO-Sal: n=12, KO-Coc: n=16). Locomotor activity was monitored for 30 min after saline or cocaine administration. A) There were no significant differences in locomotor activity between groups during the habituation period. B) Locomotor activity was significantly increased in βAdd KO mice compared to their WT siblings following repeated cocaine administration (*, p ≤ 0.05 for post hoc comparison between cocaine-treated WT and KO mice, Tukey's-HSD).
Discussion
Behavioral changes due to learning or drug exposure are thought to be the result of synaptic plasticity and neuronal adaptations that alter circuit level activity in the brain (Nestler, 2001). As a result, drug-induced structural changes in neuronal connections are mechanisms that likely underlie behavioral responses related to drug addiction (Robinson and Kolb 1999). Although the relationship between structural changes in neurons of the NAc and behaviors related to drug addiction has been investigated extensively, it is not clear how changes in spine density following repeated drug exposure contribute to behavioral responses to drugs of abuse (Robinson and Kolb 2004; Shen et al. 2009). Further, the molecular mechanisms contributing to spine remodeling following repeated drug exposure are still not well understood. In this study, we hypothesized that the cytoskeleton interacting protein βAdd might be important for spine remodeling in response to cocaine administration. In erythrocytes, βAdd contributes to membrane-cytoskeleton remodeling in a phosphorylation-dependent manner (Bennet et al, 1988). In the NAc, βAdd is phosphorylated following cocaine administration (Toda et al, 2006; Lavaur et al., 2009) suggesting that cocaine-dependent synaptic remodeling might depend on adducin signaling. We therefore used βAdd KO mice to investigate whether βAdd contributes to changes in spine density following repeated cocaine exposure.
The current set of experiments demonstrates that the density of mushroom spines is decreased at baseline in βAdd KO mice compared to their WT siblings. This decrease in spine density did not appear to disrupt behavioral sensitization to cocaine. Indeed, there was a statistically significant increase in the initial locomotor response to cocaine in βAdd KO mice that was maintained following repeated cocaine exposure. Although the current data are correlational, one possibility is that the decrease in spine density in the βAdd KO mice at baseline disinhibited the initial locomotor response to cocaine. This would be consistent with previous studies demonstrating that decreased expression of the transcription factor MEF2 decreases spine density in the NAc, prevents spine remodeling in response to cocaine, and significantly increases the locomotor response to repeated cocaine administration (Pulipparacharuvil et al. 2008). Similarly, inhibition of CDK5 by roscovitine decreases spine density in the NAc, prevents cocaine-induced increases in NAc spine density (Norrholm et al. 2003) and increases the locomotor response to repeated cocaine (Taylor et al. 2007). In contrast, knockout of the cytoskeletal protein spinophilin decreases spine remodeling overall (Feng et al. 2000), prevents cocaine-induced changes in excitatory signaling and increases the locomotor response to cocaine (Allen et al. 2006, Wu et al. 2008). Thus, it seems possible that decreased spine density in the NAc could disinhibit the locomotor response to cocaine, supporting the suggestion that spine remodeling in the NAc is a counter-adaptive homeostatic response that opposes sensitization to cocaine (Chandler and Kalivas, 2008; Pulipparacharuvil et al. 2008; Kiraly et al. 2010).
In contrast to our prediction, knockout of βAdd did not prevent the cocaine-induced increase in mushroom spine density in the NAc following repeated cocaine administration. Indeed, there was a greater increase in mushroom spines in response to cocaine in βAdd KO mice as compared to in βAdd WT mice. This suggests that βAdd may normally stabilize spines, and that loss of βAdd is permissive for greater remodeling. A recent study showed that environmental enrichment increases spine density in the hippocampus in both WT and βAdd KO mice, but that synapse stability was greatly altered in the absence of β Add (Bednarek et al 2011). β Add KO mice showed enhanced spine turn over, implying that loss of β Add increases spine lability. This partially explains that increase of mushroom spine density under cocaine challenge. Alternatively, it is also possible that α/β Add heterodimers replace α/β Add function in the NAc following constitutive knockout of the β Add protein. Future studies that manipulate all three adducin family members will be required to resolve this question.
We used two independent methods to analyze spine density in this study. Spines can be classified into categories based on spine morphology (Garcia-Lopez et al. 2006, Jedynak et al. 2007.); however, there is some disagreement on how to classify spines and consistent parameters for spine classification have not yet been established. We therefore used both NeuronStudio and IMARIS software, which use different algorithms to classify spine types, to identify common changes in spine density in the NAc of βAdd KO mice. Importantly, the two complementary approaches identified very similar patterns of spine density changes. Both methods detected a decrease in mushroom spine density at baseline in βAdd KO compared to βAdd WT mice. Overall, both methods of analysis detected consistent proportions of mushroom and long spines, and identified a very similar level and pattern of change following cocaine exposure.
In conclusion, the current study suggests that the cytoskeleton-interacting protein β Add is important for regulating mature mushroom-shaped spines under basal conditions and stabilizing cocaine-induced spines on medium spiny neurons in the NAc. These mice also showed a greater locomotor response to cocaine in βAdd KO mice as compared to their WT siblings, supporting the idea that decreased spine number at baseline could result in a greater behavioral response to cocaine challenge. Finally, in the absence of βAdd, repeated cocaine administration can still increase dendritic spine number in the NAc, although other adducin family members (α and β) may replace βAdd following constitutive knockout. Taken together, these data support a potential role for βAdd in stabilization of dendritic spines in the NAc.
Acknowledgements
This work was supported by grants DA00436 and DA14241, AA017922, and AA010983 from the National Institutes of Health.
Abbreviations
- βAdd
β adducin
- KO
knockout
- NAc
nucleus accumbens
- WT
wildtype
Footnotes
None of the authors have any conflicts of interest to declare.
References
- Allen PB, Zachariou V, Svenningsson P, Lepore AC, Centonze D, Costa C, Rossi S, Bender G, Chen G, Feng J, Snyder GL, Bernardi G, Nestler EJ, Yan Z, Calabresi P, Greengard P. Distinct roles for spinophilin and neurabin in dopamine-mediated plasticity. Neurosci. 2006;140(3):897–911. doi: 10.1016/j.neuroscience.2006.02.067. [DOI] [PubMed] [Google Scholar]
- Bednarek E, Caroni P. β-Adducin is required for stable assembly of new synapses and improved memory upon environmental enrichment. Neuron. 2011;69(6):1132–46. doi: 10.1016/j.neuron.2011.02.034. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Kalivas PW. Neuroscience: Brain's defence against cocaine. Nature. 2008;455(7214):743–744. doi: 10.1038/455743a. [DOI] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- Dong L, Chapline C, Mousseau B, Fowler L, Ramsay K, Stevens JL, Jaken S. 35H, a sequence isolated as a protein kinase C binding protein, is a novel member of the adducin family. J Biol Chem. 1995;270(43):25534–25540. doi: 10.1074/jbc.270.43.25534. [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad USA. 2000;97(16):9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Wen X, Labelle-Dumais C, Kolb B. Chronic phencyclidine treatment increases dendritic spine density in prefrontal cortex and nucleus accumbens neurons. Synapse. 2007;61(12):978–984. doi: 10.1002/syn.20452. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V, Matsuura Y, Kaibuchi K. Phosphorylation of adducin by rho-kinase plays a crucial role in cell motility. J Cell Biol. 1999;145(2):347–361. doi: 10.1083/jcb.145.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lopez P, Garcia-Marin V, Freire M. Three-dimensional reconstruction and quantitative study of a pyramidal cell of a cajal histological preparation. J Neurosci. 2006;26(44):11249–11252. doi: 10.1523/JNEUROSCI.3543-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner K, Bennett V. Modulation of spectrin-actin assembly by erythrocyte adducin. Nature. 1987;328(6128):359–362. doi: 10.1038/328359a0. [DOI] [PubMed] [Google Scholar]
- Gilligan DM, Lozovatsky L, Gwynn B, Brugnara C, Mohandas N, Peters LL. Targeted disruption of the beta adducin gene (Add2) causes red blood cell spherocytosis in mice. Proc Natl Acad USA. 1999;96(19):10717–10722. doi: 10.1073/pnas.96.19.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan DM, Sarid R, Weese J. Adducin in platelets: Activation-induced phosphorylation by PKC and proteolysis by calpain. Blood. 2002;99(7):2418–2426. doi: 10.1182/blood.v99.7.2418. [DOI] [PubMed] [Google Scholar]
- Hughes CA, Bennett V. Adducin: A physical model with implications for function in assembly of spectrin-actin complexes. J Biol Chem. 1995;270(32):18990–18996. doi: 10.1074/jbc.270.32.18990. [DOI] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci. 2007;25(3):847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Kaiser HW, O'Keefe E, Bennett V. Adducin: Ca++-dependent association with sites of cell-cell contact. J Cell Biol. 1989;109(2):557–569. doi: 10.1083/jcb.109.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Ma XM, Mazzone CM, Xin X, Mains RE, Eipper BA. Behavioral and morphological responses to cocaine require Kalirin7. Biol Psychiatry. 2010;68(3):249–55. doi: 10.1016/j.biopsych.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7(5):e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman PA, Hughes CA, Bennett V, Fowler VM. A new function for adducin. Calcium/calmodulin-regulated capping of the barbed ends of actin filaments. J Biol Chem. 1996;271(14):7986–7991. doi: 10.1074/jbc.271.14.7986. [DOI] [PubMed] [Google Scholar]
- Lavaur J, Mineur YS, Picciotto MR. The membrane cytoskeletal protein adducin is phosphorylated by protein kinase C in D1 neurons of the nucleus accumbens and dorsal striatum following cocaine administration. J Neurochem. 2009;111(5):1129–1137. doi: 10.1111/j.1471-4159.2009.06405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad USA. 2006;103(9):3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Hughes CA, Bennett V. Adducin regulation. definition of the calmodulin-binding domain and sites of phosphorylation by protein kinases A and C. J Biol Chem. 1996;271(41):25157–25166. doi: 10.1074/jbc.271.41.25157. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Li X, Bennett V. Adducin is an in vivo substrate for protein kinase C: Phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin-actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol. 1998;142(2):485–497. doi: 10.1083/jcb.142.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mische SM, Mooseker MS, Morrow JS. Erythrocyte adducin: A calmodulin-regulated actin-bundling protein that stimulates spectrin-actin binding. J Cell Biol. 1987;105(6 Pt 1):2837–2845. doi: 10.1083/jcb.105.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neurosci. 2003;116(1):19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59(4):621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenstein RL, Addy NA, Caldarone BJ, Asaka Y, Gruenbaum LM, Peters LL, Gilligan DM, Fitzsimonds RM, Picciotto MR. Impaired synaptic plasticity and learning in mice lacking beta-adducin, an actin-regulating protein. J Neurosci. 2005;25(8):2138–2145. doi: 10.1523/JNEUROSCI.3530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39(3):257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11(5):1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Shen H, Sesack SR, Toda S, Kalivas PW. Automated quantification of dendritic spine density and spine head diameter in medium spiny neurons of the nucleus accumbens. Brain Struct Funct. 2008;213(1-2):149–57. doi: 10.1007/s00429-008-0184-2. [DOI] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29(9):2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriyapperuma SP, Lozovatsky L, Ciciotte SL, Peters LL, Gilligan DM. The mouse adducin gene family: Alternative splicing and chromosomal localization. Mammalian Genome. 2000;11(1):16–23. doi: 10.1007/s003350010004. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proc Natl Acad USA. 2007;104(10):4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S, Shen HW, Peters J, Cagle S, Kalivas PW. Cocaine increases actin cycling: Effects in the reinstatement model of drug seeking. J Neurosci. 2006;26(5):1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waseem A, Palfrey HC. Erythrocyte adducin. comparison of the alpha- and beta-subunits and multiple-site phosphorylation by protein kinase C and cAMP-dependent protein kinase. Eur J Biochem. 1988;178(2):563–573. doi: 10.1111/j.1432-1033.1988.tb14483.x. [DOI] [PubMed] [Google Scholar]
- Waseem A, Palfrey HC. Identification and protein kinase C-dependent phosphorylation of alpha-adducin in human fibroblasts. J Cell Sci. 1990;96(1):93–98. doi: 10.1242/jcs.96.1.93. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Ren M, Wang H, Kim SS, Cao X, Zhuo M. Neurabin contributes to hippocampal long-term potentiation and contextual fear memory. PloS One. 2008;3(1):e1407. doi: 10.1371/journal.pone.0001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Spradling AC. Hu-li tai shao, a gene required for ring canal formation during drosophila oogenesis, encodes a homolog of adducin. Genes Devel. 1992;6(12B):2443–2454. doi: 10.1101/gad.6.12b.2443. [DOI] [PubMed] [Google Scholar]