Abstract
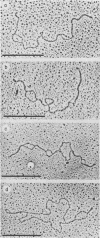
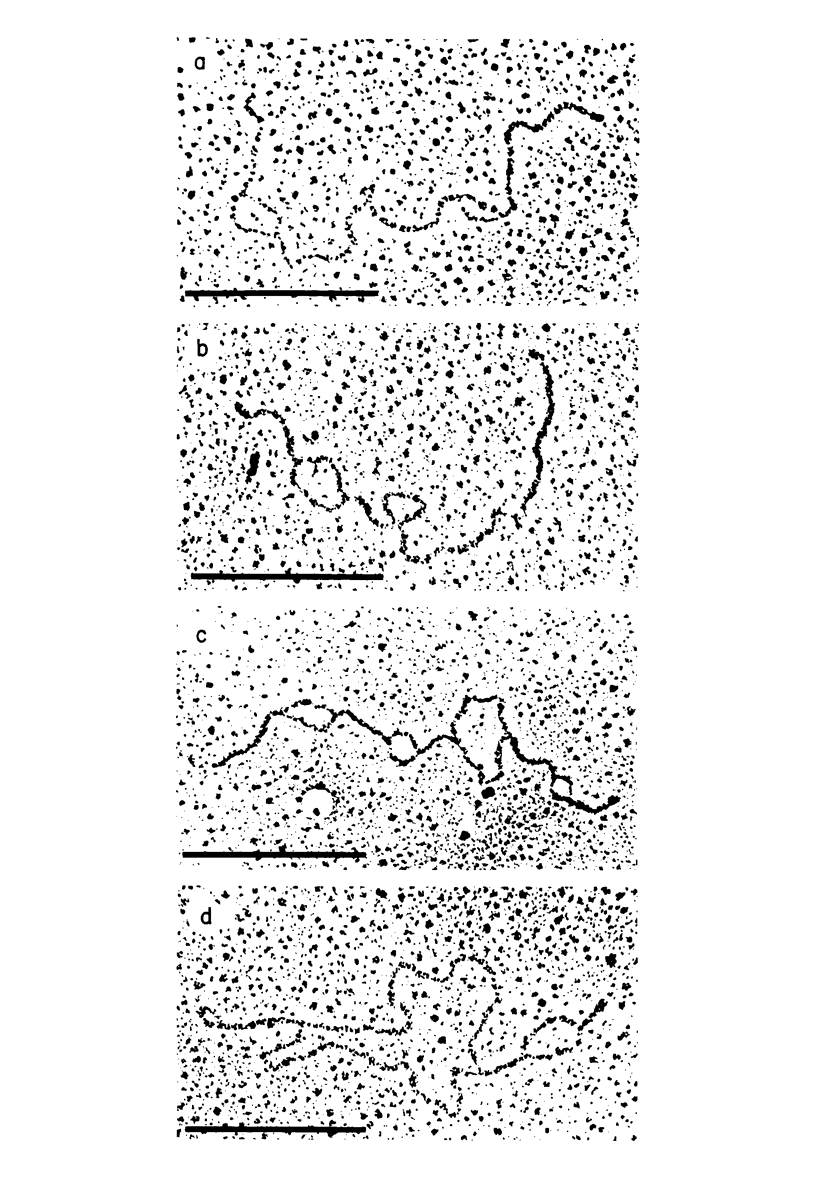
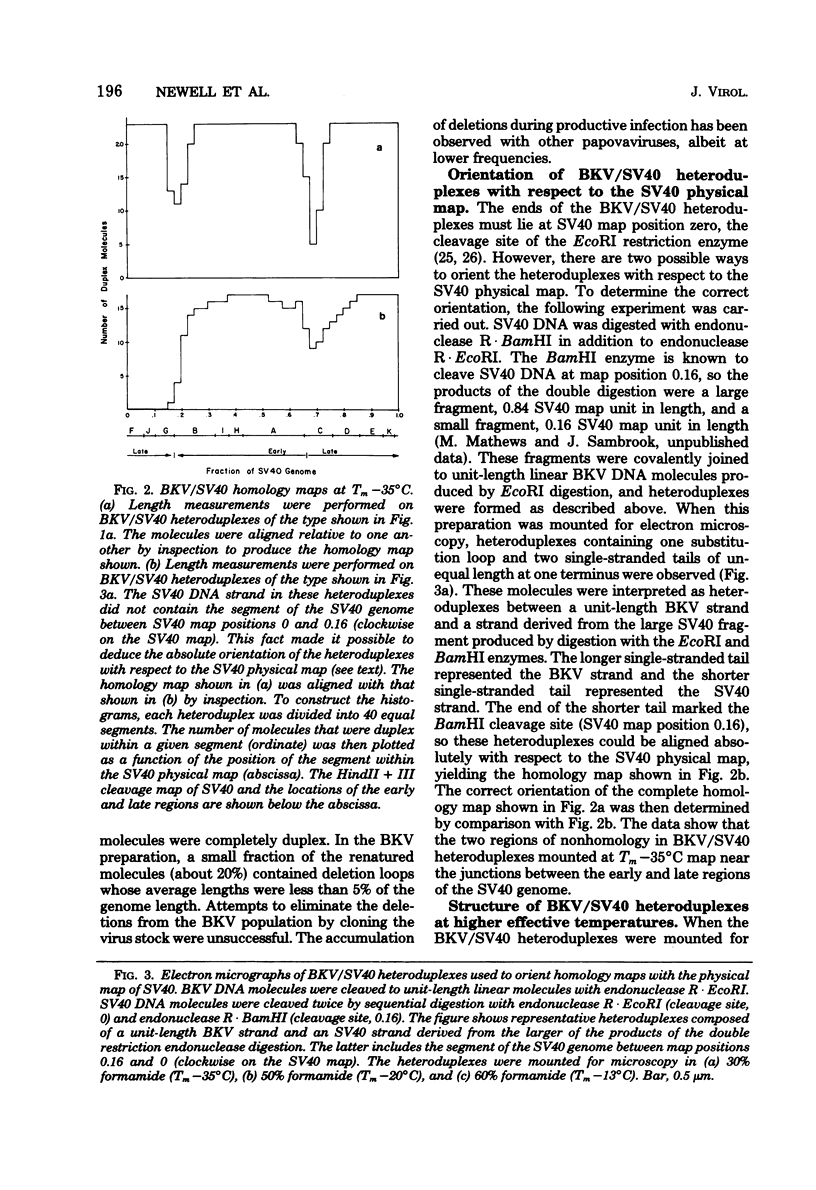
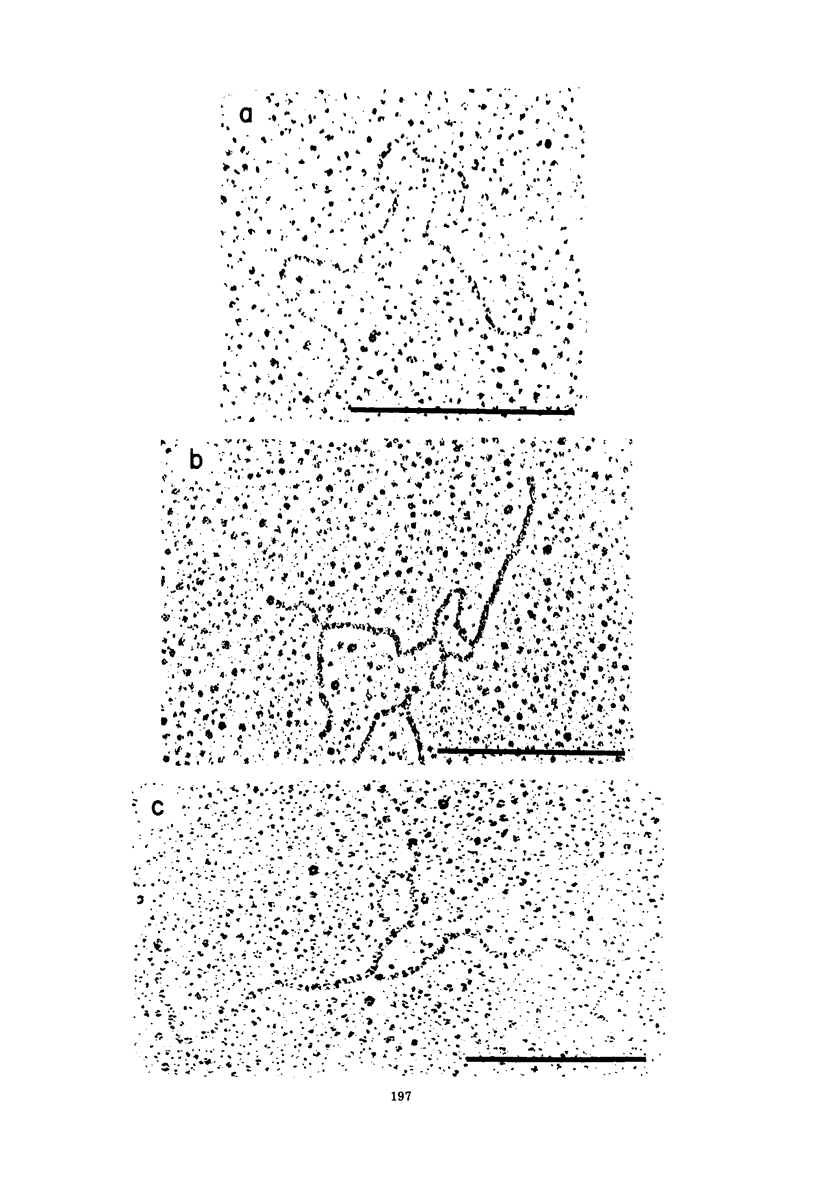
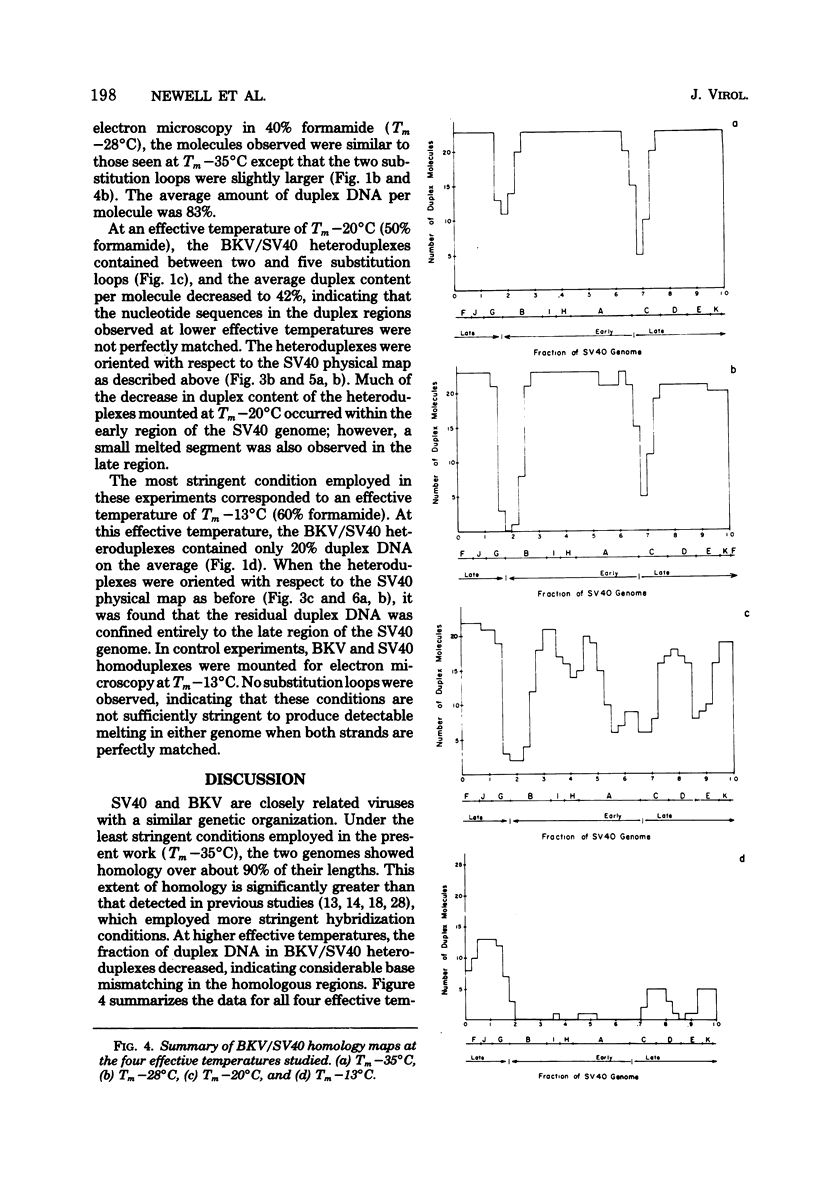
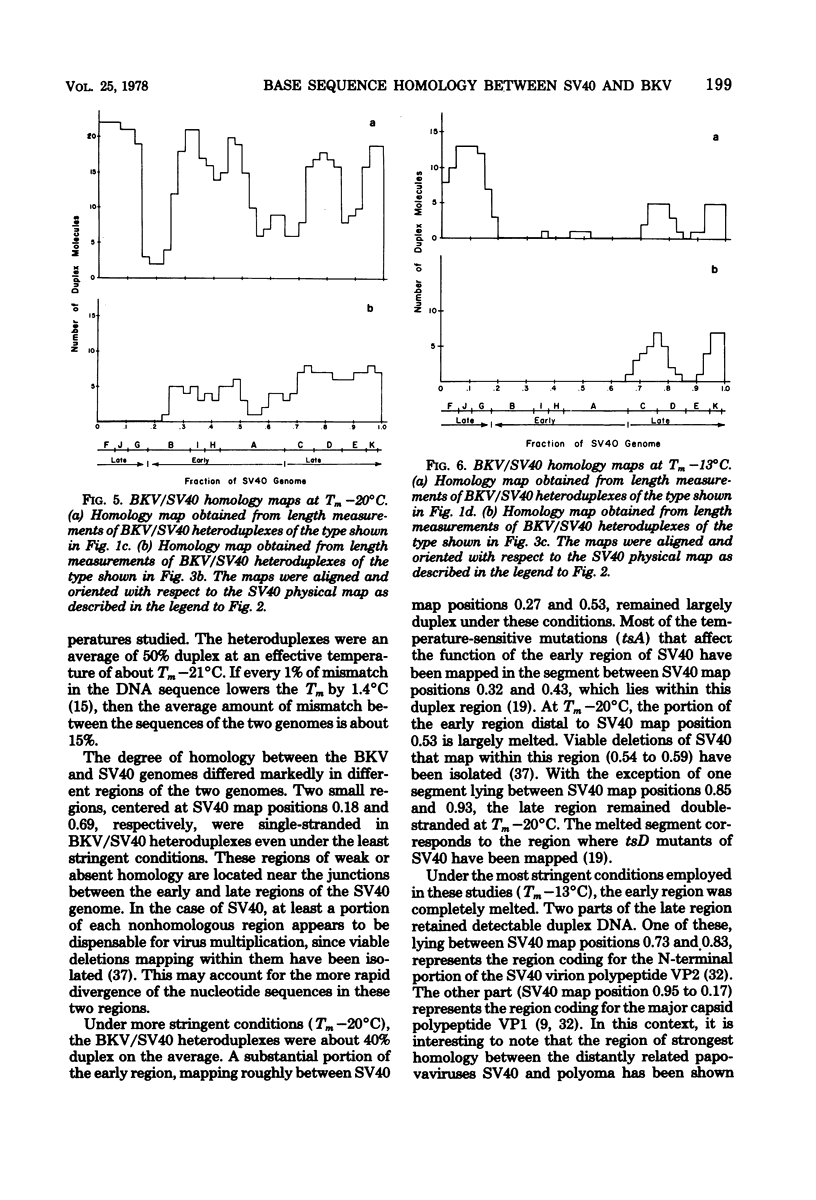
The base sequence homology between the genomes of simian virus 40 (SV40) and human papovavirus BK (BKV) was studied by the heteroduplex method of Ferguson and Davis (J. Mol. Biol. 94:135-149, 1975). When mounted for microscopy in 30% formamide (Tm-35 degrees C), BKV/SV40 heteroduplexes were an average of 92% double-stranded and contained only two small nonhomologous regions that mapped near the junctions between the early and late regions of the SV40 Genome. At higher formamide concentrations, the fraction of duplex DNA in the BKV/SV40 heteroduplexes decreased, indicating significant base mismatching in the homologous regions. The strongest regions of homology were located in the late region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- Coleman D. V., Gardner S. D., Field A. M. Human polyomavirus infection in renal allograft recipients. Br Med J. 1973 Aug 18;3(5876):371–375. doi: 10.1136/bmj.3.5876.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K., Nathans D. Specific cleavage of simian virus 40 DNA by restriction endonuclease of Hemophilus influenzae. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2913–2917. doi: 10.1073/pnas.68.12.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Hyman R. W. A study in evolution: the DNA base sequence homology between coliphages T7 and T3. J Mol Biol. 1971 Dec 14;62(2):287–301. doi: 10.1016/0022-2836(71)90428-1. [DOI] [PubMed] [Google Scholar]
- Dougherty R. M., DiStefano H. S. Isolation and characterization of a papovavirus from human urine. Proc Soc Exp Biol Med. 1974 Jun;146(2):481–487. doi: 10.3181/00379727-146-38131. [DOI] [PubMed] [Google Scholar]
- Ferguson J., Davis R. W. An electron microscopic method for studying and mapping the region of weak sequence homology between simian virus 40 and polyoma DNAs. J Mol Biol. 1975 May 15;94(2):135–149. doi: 10.1016/0022-2836(75)90073-x. [DOI] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Gardner S. D. Prevalence in England of antibody to human polyomavirus (B.k.). Br Med J. 1973 Jan 13;1(5845):77–78. doi: 10.1136/bmj.1.5845.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Khoury G., Byrne J. C., Takemoto K. K., Martin M. A. Physical map of the BK virus genome. J Virol. 1975 Oct;16(4):959–973. doi: 10.1128/jvi.16.4.959-973.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M., Mullarkey M. F., Takemoto K. K., Martin M. A. Characterization of human papovavirus BK DNA. J Virol. 1975 Jan;15(1):173–181. doi: 10.1128/jvi.15.1.173-181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman R. W., Brunovskis I., Summers W. C. DNA base sequence homology between coliphages T7 and phiII and between T3 and phiII as determined by heteroduplex mapping in the electron microscope. J Mol Biol. 1973 Jun 25;77(2):189–196. doi: 10.1016/0022-2836(73)90330-6. [DOI] [PubMed] [Google Scholar]
- Jung M., Krech U., Price P. C., Pyndiah M. N. Evidence of chronic persistent infections with polyomaviruses (BK type) in renal transplant recipients. Arch Virol. 1975;47(1):39–46. doi: 10.1007/BF01315591. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr, Levine A. S., Siegel S. Structure of two adenovirus-simian virus 40 hybrids which contain the entire SV40 genome. J Mol Biol. 1974 Oct 15;89(1):113–126. doi: 10.1016/0022-2836(74)90165-x. [DOI] [PubMed] [Google Scholar]
- Khoury G., Howley P. M., Garon C., Mullarkey M. F., Takemoto K. K., Martin M. A. Homology and relationship between the genomes of papovaviruses, BK virus and simian virus 40. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2563–2567. doi: 10.1073/pnas.72.7.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lecatsas G., Prozesky O. W. Excretion of morphological variants of human polyoma virus. Arch Virol. 1975;47(4):393–397. doi: 10.1007/BF01347981. [DOI] [PubMed] [Google Scholar]
- Lecatsas G., Prozesky O. W., van Wyk J., Els H. J. Papova virus in urine after renal transplantation. Nature. 1973 Feb 2;241(5388):343–344. doi: 10.1038/241343a0. [DOI] [PubMed] [Google Scholar]
- Mason D. H., Takemoto K. K. Complementation between BK human papovavirus and a simian virus 40 tsA mutant. J Virol. 1976 Mar;17(3):1060–1062. doi: 10.1128/jvi.17.3.1060-1062.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Cleavage of Simian virus 40 DNA at a unique site by a bacterial restriction enzyme. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3365–3369. doi: 10.1073/pnas.69.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntyjärvi R. A., Meurman O. H., Vihma L., Berglund B. A human papovavirus (B.K.), biological properties and seroepidemiology. Ann Clin Res. 1973 Oct;5(5):283–287. [PubMed] [Google Scholar]
- Näse L. M., Kärkkäinen M., Mäntyjärvi R. A. Transplantable hamster tumors induced with the BK virus. Acta Pathol Microbiol Scand B. 1975 Aug;83(4):347–352. doi: 10.1111/j.1699-0463.1975.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Robertson S. M., Padgett B. L., Walker D. L., Weisblum B. Comparison of JC and BK human papovaviruses with simian virus 40: DNA homology studies. J Virol. 1976 Aug;19(2):675–684. doi: 10.1128/jvi.19.2.675-684.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn J. E., Robertson S. M., Padgett B. L., ZuRhein G. M., Walker D. L., Weisblum B. Comparison of JC and BK human papovaviruses with simian virus 40: restriction endonuclease digestion and gel electrophoresis of resultant fragments. J Virol. 1974 Mar;13(3):614–622. doi: 10.1128/jvi.13.3.614-622.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney J. B., Jr, Narayan O. Studies of the antigenic relationships of the new human papovaviruses by electron microscopy agglutination. Infect Immun. 1973 Aug;8(2):299–300. doi: 10.1128/iai.8.2.299-300.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portolani M., Barbanti-Brodano G., Placa M. L. Malignant transformation of hamster kidney cells by BK virus. J Virol. 1975 Feb;15(2):420–422. doi: 10.1128/jvi.15.2.420-422.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt S., Mulligan R. C., Gorecki M., Roberts B. E., Rich A. Direct biochemical mapping of eukaryotic viral DNA by means of a linked transcription-translation cell-free system. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2747–2751. doi: 10.1073/pnas.73.8.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Daniel R. W., Strandberg J. D. Sarcoma in a hamster inoculated with BK virus, a human papovavirus. J Natl Cancer Inst. 1975 Apr;54(4):945–950. [PubMed] [Google Scholar]
- Shah K. V., Daniel R. W., Warszawski R. M. High prevalence of antibodies to BK virus, an SV40-related papovavirus, in residents of Maryland. J Infect Dis. 1973 Dec;128(6):784–787. doi: 10.1093/infdis/128.6.784. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Ozer H. L., Ghazey H. N., Kelly T. J., Jr Common structural antigen of papovaviruses of the simian virus 40-polyoma subgroup. J Virol. 1977 Jan;21(1):179–186. doi: 10.1128/jvi.21.1.179-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Takemoto K. K., Martin M. A. Relationship between the methionine tryptic peptides of simian virus 40 and BK virus tumor antigens. J Virol. 1977 Oct;24(1):319–325. doi: 10.1128/jvi.24.1.319-325.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Mullarkey M. F. Human papovavirus, BK strain: biological studies including antigenic relationship to simian virus 40. J Virol. 1973 Sep;12(3):625–631. doi: 10.1128/jvi.12.3.625-631.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Rabson A. S., Mullarkey M. F., Blaese R. M., Garon C. F., Nelson D. Isolation of papovavirus from brain tumor and urine of a patient with Wiskott-Aldrich syndrome. J Natl Cancer Inst. 1974 Nov;53(5):1205–1207. doi: 10.1093/jnci/53.5.1205. [DOI] [PubMed] [Google Scholar]