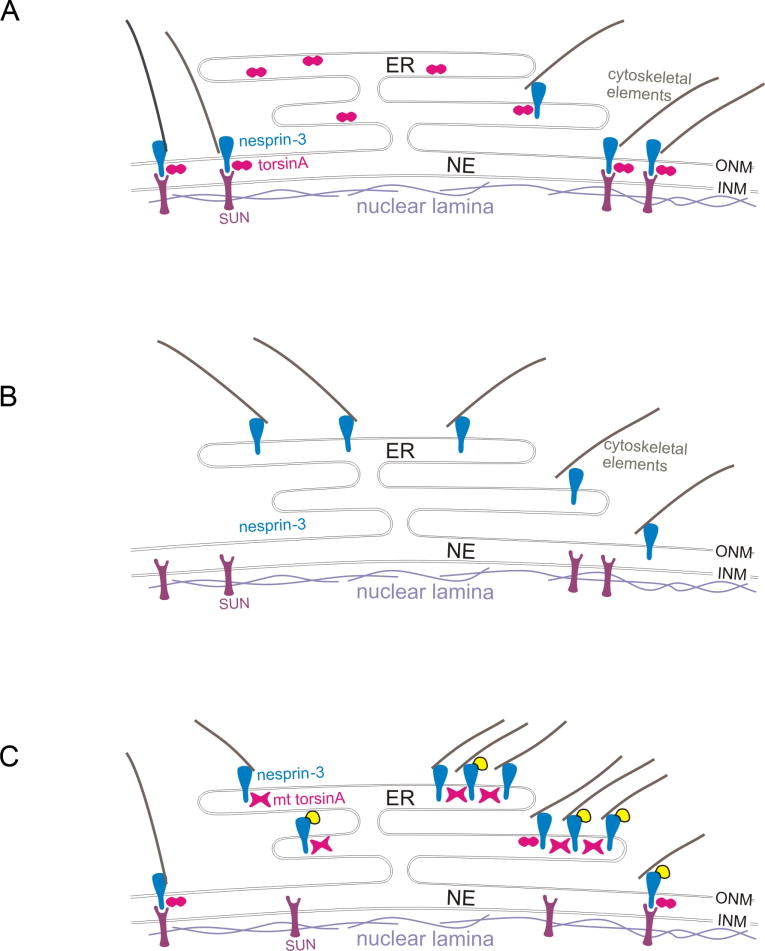
Fig. 10.
Theoretical consequences of torsinA status for nesprin-SUN interactions. Nesprins span the outer nuclear membrane (ONM) and interact with cytoskeletal elements in the cytoplasm and with inner nuclear membrane (INM) proteins, such as SUNs, in the lumen of the nuclear envelope (NE). TorsinA is hypothesized to act as an AAA+ protein in the disassembly/reassembly of these nesprin-SUN interactions, for example, during movement of the nucleus in cell migration. (A) In wild-type cells, nesprins and associated cytoskeletal elements are localized to the ONM by interactions with INM proteins, such as SUNs, and this interaction (at least for nesprin-3) is modulated by torsinA (depicted as an oligomer in cross-section, two circles). (B) In torsinA-null cells, nesprin-3 and linked cytoskeletal elements are located predominantly in association with the endoplasmic reticulum (ER) (see Fig. 6), as the association of nesprins with INM proteins is predicted to be compromised. (C) Cells from DYT1 subjects express both torsinA and torsinAΔE, with the mutant (mt) torsinA hypothesized to form inactive oligomers with torsinA (depicted as X-shaped structures). When YFP-nesprin-3 is overexpressed in DYT1 cells, torsinA oligomers containing torsinAΔE, which binds more tightly than torsinA to nesprins, reduce the interaction of YFP-nesprin-3 with INM proteins, such that YFP-nesprin-3 accumulates in the ER, where, together with torsinA/torsinAΔE and associated cytoskeletal elements, it forms globular structures (see Fig. 2).