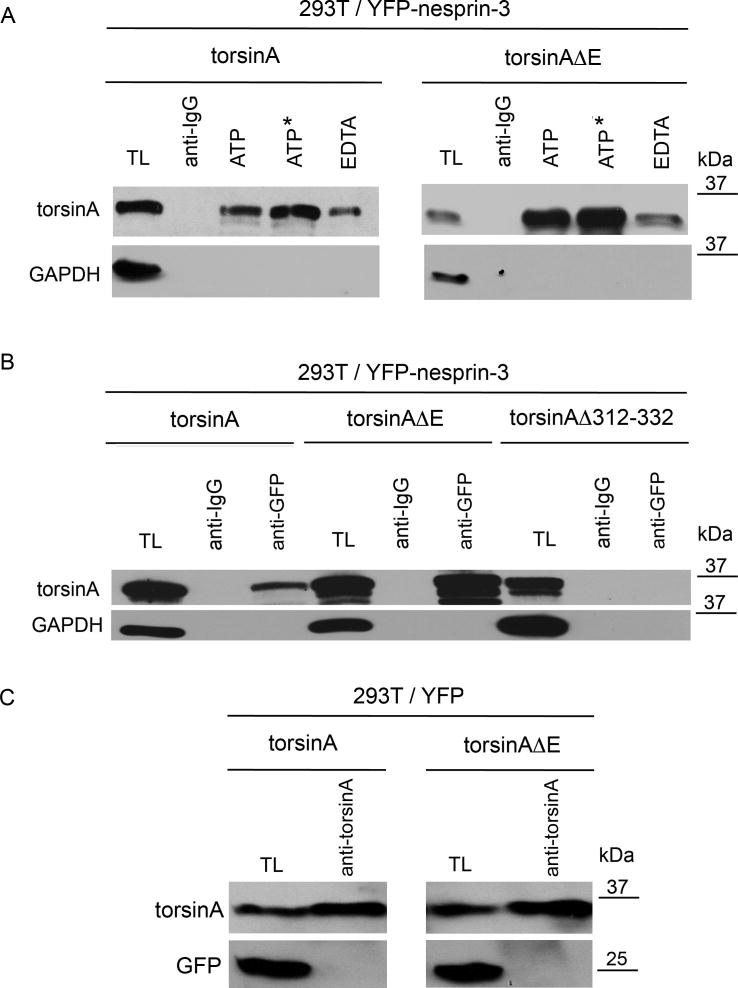
Fig. 3.
Association of torsinA and torsinAΔE with nesprin-3. (A) Human 293T cells were co-transfected with expression cassettes for YFP-nesprin and either torsinA or torsinAΔE. Immunoprecipitations were carried out in the presence of 1 mM ATP, non-hydrolysable ATP or EDTA (the latter with no added ATP) in RIPA buffer with antibody to GFP or with anti-rabbit IgG as control. Immunoprecipitated proteins were resolved by SDS-PAGE and immunoblotted with antibodies to torsinA (D-M2A8) or GAPDH. On average, threefold more torsinAΔE was immunoprecipitated than torsinA (3.0±0.88, n=3, P<0.0001, ANOVA). (B) Experiments were carried out as in A except that transfections were with expression cassettes for torsinA, torsinAΔE or torsinAΔ312–322. The additional bands under the predominant 37-kDa torsinA band presumably represent the non- and partially glycosylated forms seen in other studies (Hewett et al., 2004). (C) To verify that torsinA and torsinAΔE bind to nesprin-3 and not to YFP, 293T cells were co-transfected with expression cassettes for YFP and either torsinA or torsinAΔE. Immunoprecipitations were carried out in the presence of 1 mM ATP in RIPA buffer with antibody to torsinA. Immunoprecipitated proteins were resolved by SDS-PAGE and immunoblotted with antibodies to torsinA (D-M2A8) or GFP.