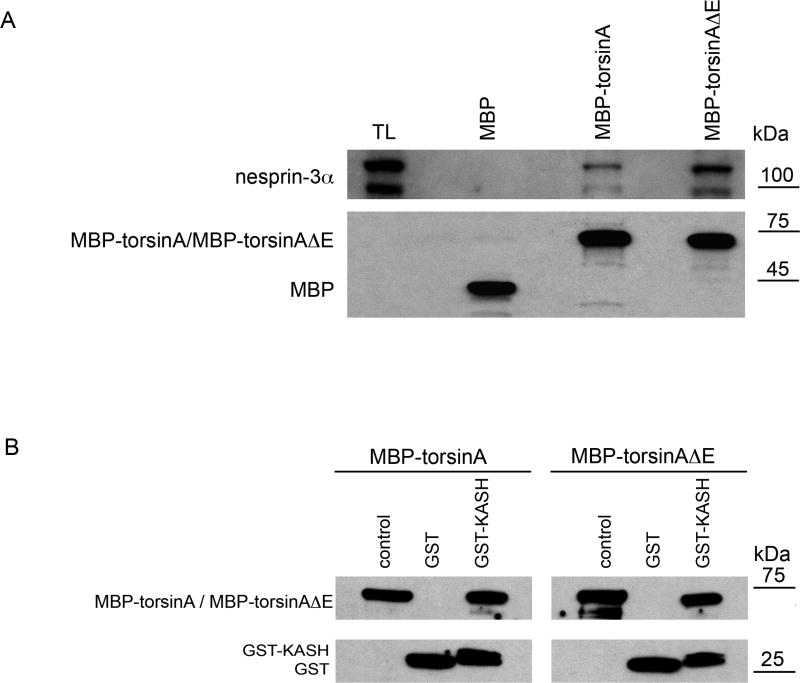
Fig. 4.
Binding of MBP-torsinA and MBP-torsinAΔE recombinant proteins to nesprin-3α and GST KASH. (A) MBP, MBP-torsinA or MBP-torsinAΔE fusion proteins (Hewett et al., 2003) (8 μg) were bound to amylase resin (New England Biolabs) and incubated overnight at 4°C with lysates of wild-type MFs (500 μl protein in 1 ml) in RIPA buffer containing 1 mM ATP and 3 mM MgCl2. Beads were washed five times with RIPA buffer and proteins bound to beads resolved by SDS-PAGE and immunoblotted with antibodies to nesprin-3α (top) and MBP (bottom), with relative binding assessed as in Fig. 1. MBP-torsinAΔE consistently pulled down ~threefold more nesprin-3α than did MBP-torsinA (3.0±0.3, n=3, P<0.003, ANOVA). The lower, immunoreactive nesprin-3α band is a degradation product, also seen by Wilhelmsen et al. (Wilhelmsen et al., 2005). (B) GST or GST-KASH fusion protein (10 μg) bound to glutathione-Sepharose beads was incubated with 10 μg of MBP-torsinA or MBP-torsinAΔE in 500 μl PBS containing 1 mM ATP and 3 mM MgCl2 for 1 hour at 4°C. Unbound material was removed by centrifugation and the beads washed five times with 0.1% NP40 buffer followed by an additional wash with PBS. The proteins bound to the beads were resolved by SDS-PAGE and visualized by western blotting with antibodies to MBP and GST.