Figure 3. The R460D D462R mutant reduces receptor dimerization, enhancing GR-mediated transrepression at theTSLP nGRE.
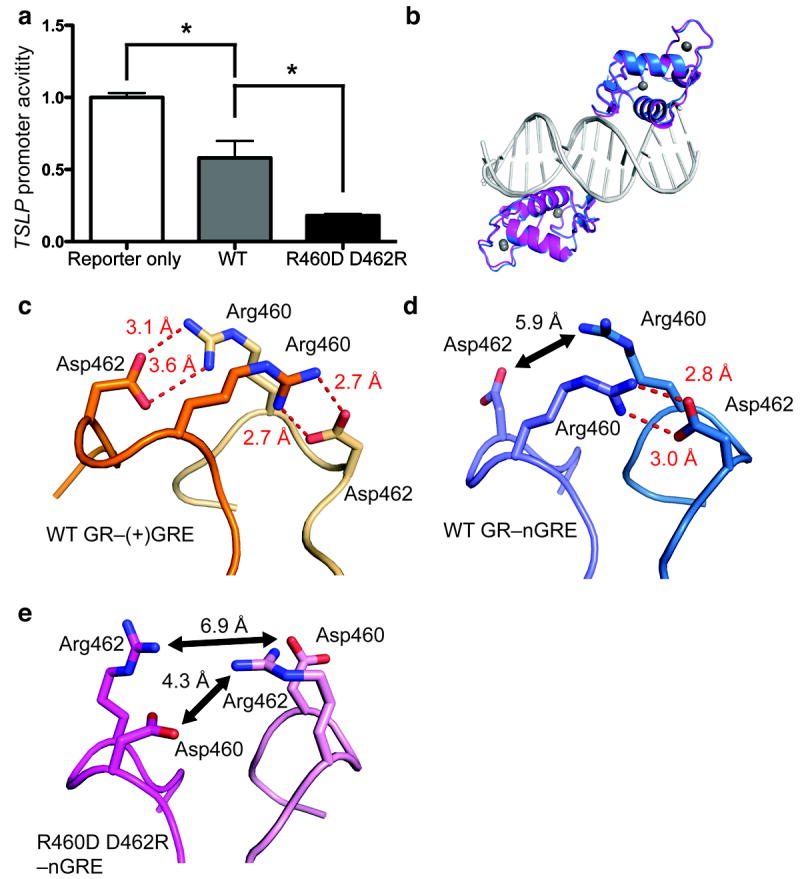
(a) Transfection of the R460D D462R mutant in HeLa cells potentiates downregulation of a constitutively active TSLP promoter compared to WT GR (5 ng each). Data are represented as the mean ± s.e.m of two independent experiments with five internal replicates each. (b) Superposition of GR (blue) and the GR R460D D462R mutant (magenta) bound to the TSLP nGRE (gray). (c) When bound to a (+)GRE element, Arg460 and Asp462 form two intermolecular salt bridges (red dashes) across the homodimer interface (PDB 3FYL). In the GR nGRE structure, crystal-packing interactions require the formation of a pseudo-continuous DNA helix and promote the formation of a pseudo-GR dimer across a two-fold symmetry axis (d). These interactions are necessary for crystal formation, but are not possible in solution-based binding assays. The R460D D462R mutation (e) ablates key dimerization contacts between GR monomers, disrupting symmetry-imposed dimerization contacts.
