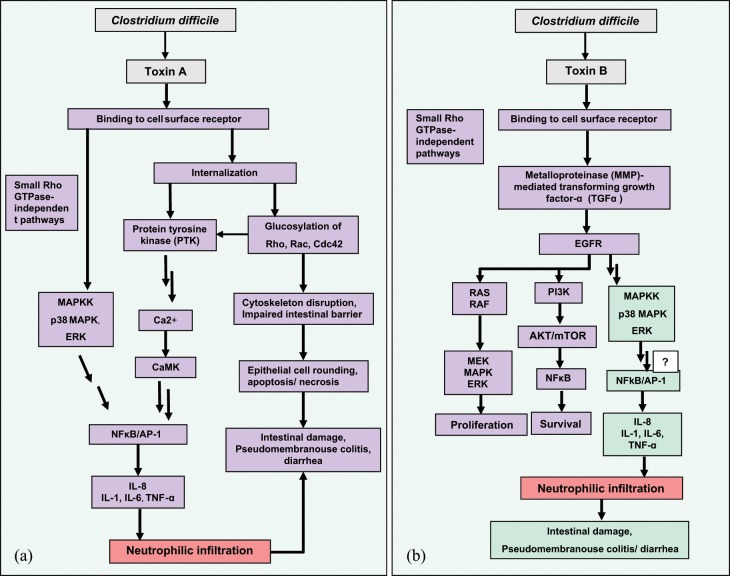
Figure 1.
(a) Mechanism of Clostridium difficile toxin A-mediated inflammation. After receptor binding, toxin A is internalized into target cells (Rho-dependent signaling) and induced interleukin (IL)-8 expression at the level of gene transcription; this effect occurred through a mechanism requiring intracellular Ca2+, calmodulin, calcium/calmodulin-dependent kinase (CaMK), protein tyrosine kinase (PTK), and nuclear factor κB (NFκB), and activator protein 1 (AP-1) activation. The Rho-independent signaling pathway induced by toxin A in human monocytic cells occurred through a mechanism requiring interaction with surface receptor, p38 mitogen-activated protein (MAP) kinase and extracellular signal-regulated kinase (ERK) activation. Kinase activation leads to NFκB translocation and IL-8 gene expression in target cells. (b). Mechanism of C. difficile toxin B-mediated inflammation. Toxin B through an unidentified membrane receptor activates the ERK-MAP kinase pathway via signaling dependent on epidermal growth factor receptor (EGFR). Metalloproteinase (MMP)-mediated transforming growth factor α (TGFα) is needed for phosphorylation of EGFR. Toxin B-mediated EGFR and ERK-MAP kinase activation and IL-8 gene expression are independent of Rho glucosylation. NFκB activation is not linked to toxin B-mediated IL-8 expression gene, but other unidentified signaling pathways may be involved in this process. EGFR activation of the MAP kinase-associated pathway has also been associated with cell proliferation and tissue repair. This process recruits the RAS-RAF-MAP kinase pathway, resulting in increased cellular proliferation. EFGR signaling also activates the phosphatidylinositol 3 kinase (PI3K)-serine threonine kinase Akt (AKT)-mammalian target of rapamycin (mTOR) pathway, which activates the major cellular survival and antiapoptosis signals via activating NFκB. Color legend: bacterial cell (gray); epithelial cell (green/violet); immune cells (red).