Summary
Background
Association between preoperative perfusion pattern and reperfusion after carotid endarterectomy (CEA) is an important yet unexplored topic. Therefore, the aim of our study was to determine whether 99mTc-ECD single-photon emission computed tomography (SPECT) performed before carotid endarterectomy in patients with internal carotid artery (ICA) stenosis may be helpful in predicting early perfusion changes after revascularization.
Material/Methods
The examined group consisted of 30 patients (mean age 67.4±9.6 years) with ICA stenosis who underwent CEA. Infarction was demonstrated on computed tomography (CT) in 12 cases. Brain perfusion SPECT was performed 1–3 days before CEA and 3–5 days after the surgery. Voxel-based analysis was carried out with Brain SPECT Quantification software. For evaluation of preoperative interhemispheric asymmetry of perfusion, the percentage asymmetry index (AI) was calculated. For comparison of perfusion before and after CEA, the percentage relative difference (RD) was computed.
Results
Before CEA, cerebral hypoperfusion was seen in 26 cases, including 15 participants with normal CT. After CEA, the following changes of perfusion were observed: perfusion increase n=18 (ipsilateral and bilateral), deterioration n=1, mixed patterns n=2, no change n=9. In patients with preoperative ipsilateral hypoperfusion and perfusion increase after CEA, AI correlated significantly with RD (r=0.48, p=0.04).
Conclusions
Our results suggest that perfusion increase 3–5 days after CEA is higher in patients with greater ipsilateral asymmetry index. Evaluation of preoperative AI may help to identify patients in whom rapid reperfusion is more likely.
Keywords: brain perfusion SPECT, voxel-based analysis, internal carotid artery stenosis, endarterectomy, interhemispheric perfusion asymmetry
Background
In addition to the removal of the embolic source, protecting the patient from cerebral embolism, partial or complete normalization of cerebral hemodynamics is accepted as a beneficial outcome from CEA. However, hemodynamic changes associated with revascularization of ICA stenosis or occlusion are not completely understood. Relations between preoperative impairment of hemodynamic parameters and risk of postoperative ischemic stroke or hyperperfusion syndrome, and long-term predictive value of early hemodynamic changes after treatment, especially in the context of cognitive dysfunction, still need clarification. These issues have recently begun to be successfully addressed by means of functional imaging techniques such as perfusion computed tomography (PCT) [1], perfusion-weighted imaging (PWI) [2], positron emission tomography (PET) [3,4] and SPECT [5–7].
Association between preoperative perfusion pattern and reperfusion after CEA is an important yet unexplored topic, and understanding of this association may be of assistance to the vascular surgeon in daily clinical practice. Therefore, the aim of our study was to determine whether 99mTc-ECD SPECT with voxel-based analysis performed before CEA may be helpful in predicting early perfusion changes after revascularization. We focused on evaluation of preoperative hypoperfusion and its relationship with perfusion changes after the treatment in a non-selected, heterogeneous group of patients with ICA stenosis, who underwent CEA.
Material and Methods
Patients
The examined group consisted of 30 patients (23 males, 7 females) with ICA stenosis who underwent CEA. Mean age was 67.4±9.6 years (range, 44 to 80 years). The studied population included consecutive patients, who gave consent to participate in our study. The degree of carotid stenosis was assessed by Doppler ultrasound. Percentage of stenosis ipsilateral to CEA ranged from 70% to 99% (mean 81.8%±6.1). Twenty-one patients had contralateral stenosis (50% to 90%; mean 63.2% ±12.9). In 2 patients contralateral ICA was occluded.
Neurological examination was performed before and after CEA. Permanent neurological deficit in patients with a history of ischemic stroke was present in 8 cases. Fourteen patients sustained transient ischemic attacks (TIA); 8 cases were asymptomatic. Hypertension was diagnosed in 24 patients, and 22 had coronary heart disease.
Preoperative brain CT was performed on all subjects. Infarction was demonstrated on CT scans (CT+) in 12 patients (8 stroke patients, 3 cases with TIA and 1 asymptomatic). CT was normal (CT−) in 18 cases (7 asymptomatic and 11 with TIA). CT results and clinical manifestations of examined patients are listed in Table 1.
Table 1.
Clinical data and scintigraphic findings in examined patients.
| Case no | Age | Sex | Computed tomography | Clinical manifestation | Hypoperfusion before CEA** | Perfusion changes after CEA** |
|---|---|---|---|---|---|---|
| 1 | 63 | M | Abnormal | Stroke | Ipsilateral | Mixed pattern |
| 2 | 77 | F | Abnormal | Stroke | Ipsilateral | No change |
| 3 | 67 | M | Abnormal | TIA* | Ipsilateral | Ipsilateral increase |
| 4 | 77 | M | Abnormal | Stroke | Ipsilateral | Ipsilateral increase |
| 5 | 62 | M | Abnormal | Asymptomatic | Ipsilateral | No change |
| 6 | 76 | M | Abnormal | Stroke | Ipsilateral | Bilateral increase |
| 7 | 71 | M | Abnormal | Stroke | Ipsilateral | Ipsilateral increase |
| 8 | 44 | M | Abnormal | Stroke | Bilateral | Bilateral increase |
| 9 | 59 | M | Abnormal | Stroke | Ipsilateral | Ipsilateral increase |
| 10 | 73 | M | Abnormal | Stroke | Bilateral | Ipsilateral increase |
| 11 | 68 | M | Abnormal | TIA* | Ipsilateral | Mixed pattern |
| 12 | 79 | M | Abnormal | TIA* | – | No change |
| 13 | 73 | M | Normal | Asymptomatic | Bilateral | Bilateral increase |
| 14 | 78 | M | Normal | TIA* | Ipsilateral | No change |
| 15 | 78 | M | Normal | Asymptomatic | Bilateral | No change |
| 16 | 75 | M | Normal | TIA* | – | No change |
| 17 | 72 | M | Normal | Asymptomatic | – | No change |
| 18 | 57 | F | Normal | TIA* | Ipsilateral | Ipsilateral increase |
| 19 | 59 | M | Normal | Asymptomatic | Bilateral | Bilateral increase |
| 20 | 44 | M | Normal | TIA* | Bilateral | Bilateral increase |
| 21 | 55 | M | Normal | Asymptomatic | Bilateral | Bilateral increase |
| 22 | 65 | M | Normal | Asymptomatic | Bilateral | Contralateral deterioration |
| 23 | 76 | F | Normal | TIA* | Bilateral | Ipsilateral increase |
| 24 | 59 | M | Normal | Asymptomatic | Bilateral | Bilateral increase |
| 25 | 63 | F | Normal | TIA* | Contralateral | Bilateral increase |
| 26 | 67 | M | Normal | TIA* | Ipsilateral | No change |
| 27 | 80 | F | Normal | TIA* | Ipsilateral | Ipsilateral increase |
| 28 | 65 | F | Normal | TIA* | – | No change |
| 29 | 72 | M | Normal | TIA* | Ipsilateral | Ipsilateral increase |
| 30 | 68 | F | Normal | TIA* | Ipsilateral | Ipsilateral increase |
Transient ischemic attacks;
Carotid endarterectomy.
The study protocol was approved by the local ethics committee and informed consent was obtained from all participants.
Data acquisition
Brain perfusion SPECT was performed 1–3 days before CEA and 3–5 days after the surgery using 99mTc-ethyl cysteinate dimmer (99mTc-ECD) (Department of Radiopharmaceuticals Production, Medical University in Lodz, Poland). The radiopharmaceutical was injected intravenously at a dose of 740 MBq in a quiet environment, with the patients in the supine position and with eyes open. SPECT acquisition was carried out 20–30 min after injection, using a rotating, double-head, large field of view gamma camera (Varicam, GE Medical Systems), equipped with low-energy, high-resolution collimators. The data were collected in a 128×128 matrix through 360° rotation at 3° intervals for 25 s per view. Reconstruction of transaxial slices was performed by filtered back projection (Metz filter power, 3.00; full width at half maximum, 10 mm) with subsequent attenuation correction using the Chang method (attenuation coefficient 0.11).
Data analysis
SPECT scans were assessed visually and semiquantitatively. Semiquantitative evaluation was carried out with Brain SPECT Quantification software (Compart Medical Systems, Poland). The software automatically matches individual SPECT with the template (norm). Such a transformation process, defined as spatial normalization, consists of scaling, shifting and rotation. The norm provided by Compart Medical System was created by averaging scans of 11 normal patients. Before registration to the template, the patient study was interpolated to the same resolution as the norm (2×2×2 mm). The next steps were study matching and count normalization to the whole brain, after which the voxel-based analysis was possible.
In hypoperfused regions of the basal SPECT, the percentage inter-hemispheric asymmetry values were ascertained. Asymmetry index (AI) was calculated in each voxel, using the formula:
where C and I represent counts in the contralateral and ipsilateral symmetrical voxels, respectively. This step was followed by cluster analysis. Abnormalities were discerned by a 3D region-growing algorithm which compares voxels in the image to the corresponding limits (cut-off values). These values can be set by the user in a program configuration. Contiguous regions are identified for which these conditions are met. Clusters of abnormal regions are listed and reported with respect to the absolute cluster size in ml-cluster volume (CV), severity (the mean percentage of AI in the cluster region) and location. The cut-off values were set as follows: AI higher than 10% in a cluster volume greater than 10 ml. In the present study, AI is always a positive number, expressing the severity of ipsilateral or contralateral hypoperfusion in relation to the opposite hemisphere. Cluster analysis of hypoperfused regions in both hemispheres is presented in Figure 1 (B, D) and in the ipsilateral hemisphere in Figure 2 (C).
Figure 1.
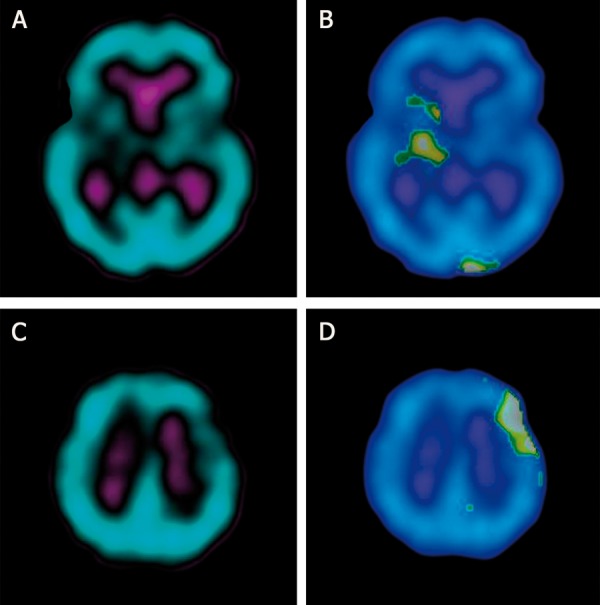
Example of bilateral hypoperfusion on preoperative SPECT in patient no 23. (A) region of hypoperfusion in the contralateral hemisphere (right basal ganglia), (B) cluster analysis of contralateral hypoperfusion (asymmetry index=13%, cluster volume=16 ml), (C) region of hypoperfusion in the ipsitralateral hemisphere (left frontal lobe), (D) cluster analysis of ipsitralateral hypoperfusion (asymmetry index=15.3%, cluster volume=22 ml).
Figure 2.
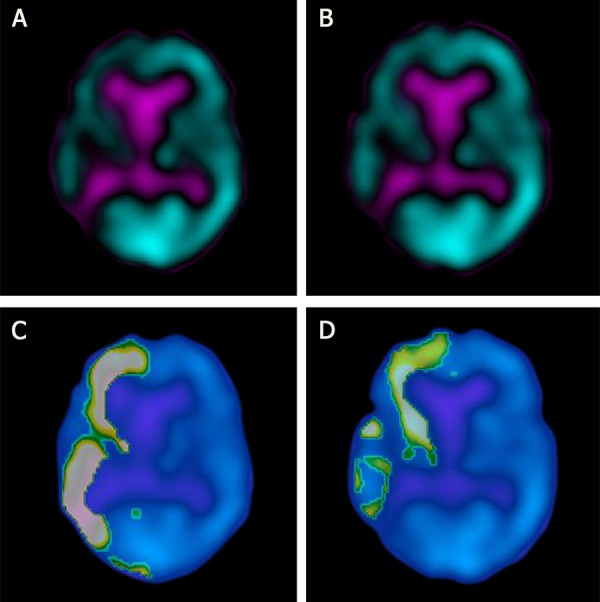
Example of voxel-based analysis of preoperative ipsilateral hypoperfusion (right hemisphere) and perfusion increase after carotid endarterectomy in patient no 9: (A) preoperative SPECT, (B) postoperative SPECT, (C) cluster analysis of preoperative SPECT (asymmetry index=23.7%, cluster volume=233 ml), (D) cluster analysis of postoperative SPECT (relative difference=18.5%:, cluster volume=175 ml).
For comparison of perfusion before and after CEA, both baseline and postoperative studies were registered to the template. The relative difference (RD) was computed in each voxel, using the formula:
where P and B represent counts in the postoperative and baseline studies, respectively. Cluster analysis applied to RD was carried out in the same way as described above. The cut-off values were set as RD higher than 10% in a CV greater than 10 ml. The cut-off percentage value of RD may be set by the user in a program configuration as a positive or negative number to find perfusion improvement or deterioration. Clusters of abnormal regions of perfusion changes after CEA are listed and reported with respect to volume (CV), severity (the mean percentage of RD in the cluster region) and location. Example of cluster analysis of ipsilateral perfusion increase after CEA is shown on Figure 2 (D).
Statistical analysis
All data were expressed as mean ± standard deviation (SD). The correlations between AI and selected parameters were assessed by Spearman’s Rank Correlation Test. A probability equal or less than 0.05 was considered statistically significant. Statistical analysis was performed using the software STATISTICA (StatSoft, Poland).
CEA
CEA was performed under general anesthesia. Sixteen patients underwent surgery on the left side and 14 on the right side. No complications were observed after the treatment. Neurological status of the patients did not change between CEA and control SPECT.
Results
SPECT results in examined patients are summarized in Table 1.
Before CEA cerebral perfusion was normal in 4 (13%) cases (1 patient CT+ and 3 patients CT−). In 26 (87%) cases SPECT was abnormal, including 15 participants with normal CT (6 asymptomatic and 9 with TIA). Hypoperfusion in only ipsilateral hemisphere was seen in 15 cases (9 patients CT+ and 6 patients CT−); in only contralateral hemisphere in 1 case (CT−). Bilateral focal defects of perfusion were detected in 10 cases (2 patients CT+ and 8 patients CT−). Volume and severity of the perfusion defects are contained in Table 2.
Table 2.
Severity and volume of hypoperfusion before carotid endarterectomy.
| Localization of hypoperfusion | n | Asymmetry Index in% | Cluster Volume in ml | ||
|---|---|---|---|---|---|
| Range | Mean ±SD | Range | Mean ±SD | ||
| Ipsilateral hemisphere | 25 | 13–31.3 | 17.8±3.6 | 12–173 | 65.5±46.9 |
| Contralateral hemisphere | 11 | 15.3–24 | 18.6±3.1 | 18.5–233 | 72.3±67.2 |
After CEA, several different patterns of perfusion redistribution were observed. Perfusion increase was seen in 18 (60%) cases. The increase was ipsilateral to CEA in 10 cases (5 patients CT+ and 5 patients CT−) and bilateral in 8 cases (2 patients CT+ and 6 patients CT−). In 1 (3%) case (CT−), perfusion deteriorated in the contralateral hemisphere. Mixed patterns of perfusion redistribution were observed in 2 (7%) cases (CT+): regions of increase and deterioration in the ipsilateral hemisphere in the first patient (case no 1) and in both hemispheres in the second patient (case no. 11). In 9 (30%) cases (3 patients CT+ and 6 patients CT−) postoperative perfusion did not change. Volume and severity of perfusion changes are contained in Table 3.
Table 3.
Severity and volume of perfusion changes after carotid endarterectomy.
| Localization of perfusion change after CEA* | n | Relative Difference in% | Cluster Volume in ml | ||
|---|---|---|---|---|---|
| Range | Mean ±SD | Range | Mean ±SD | ||
| Ipsilateral increase | 20 | 12.1–30 | 17.4±4.2 | 10.2–194.9 | 56.7±51.5 |
| Contralateral increase | 9 | 14.2–19.1 | 16.5±1.7 | 10.2–194.9 | 82.9±67.4 |
| Ipsilateral decrease | 2 | 14.4–16 | 15.2±1.1 | 16.6–17 | 16.8±0,3 |
| Contralateral decrease | 2 | 13.8–15 | 14.4±0.8 | 24.6–28.1 | 26.3±2.5 |
Carotid endarterectomy.
In the ipsilateral hemisphere, AI correlated significantly with the degree of stenosis (r=0.39, p=0.05, n=25) and with RD of ipsilateral perfusion increase (r=0.48, p=0.04, n=19). The relationship between the degree of ipsilateral stenosis and RD of ipsilateral perfusion increase was not statistically significant (r=0.11; p=0.64, n=20).
In the contralateral hemisphere, correlation between AI and RD of perfusion improvement was not statistically significant (r=0.42, p=0.23, n=7).
Discussion
The present study detected impaired perfusion before CEA in as many as 26 out of 30 patients (87%), including 15 participants with normal CT. The SPECT scan has the capability of demonstrating ischemic areas not shown by CT [8]. Hypoperfusion was diagnosed ipsilaterally, contralaterally or bilaterally. Our data support the results of several authors, using various imaging methods: SPECT [9], PET [3], PCT [10] and PWI [11].
Contrary to the findings of Sfyroeras et al. [12], we determined weak but statistically significant correlation (r=0.39, p=0.05) between AI and percentage of stenosis in the ipsilateral hemisphere. Those authors explained the lack of correlation in their work by vasodilatation, autoregulatory mechanisms, and collateral circulation that play important roles in perfusion distribution apart from the degree of the artery narrowing. Our results, based on a more precise semiquantitative method (voxel-based versus region of interest, discussed below) confirm findings of Vanninen et al. [13], who observed a high linear correlation between perfusion heterogeneity index and ipsilateral carotid stenosis degree (r=0.74, p=0.003). Perfusion heterogeneity index was calculated as the coefficient of variation of 44 cerebral regions.
Perfusion increase after CEA was observed by the present study in the majority of participants (60%), including those with and without morphological lesions. Other authors have documented quite similar changes, despite differences in patient selection, time interval between CEA and control imaging or methods of visual or semiquantitative data analysis. We corroborate findings of Tawes et al. [8], who found perfusion normalization on post-CEA 99mTc-HMPAO SPECT scans in 48 of 74 symptomatic and asymptomatic patients. Bilateral increase of cerebral perfusion, especially at the side of more expressed damage, and subsequent diminution of preoperative interhemispheric asymmetry of perfusion was reported by Lishmanov et al. [14] in a group of 36 patients with different degrees of carotid stenosis. Otte et al. [15] described significant improvement of brain perfusion after CEA, combined with normalization of interhemispheric perfusion asymmetry in a study of 74 patients with unilateral and symptomatic stenosis. Brain perfusion was stable over a 12-month period post-surgery.
Hemodynamic effect of CEA on cerebral perfusion was also studied by PET. Our findings agree with Hino et al. [3], who observed reduction of perfusion and metabolism in the hemispheres ipsilateral and contralateral to symptomatic unilateral ICA stenosis. CEA normalized measured parameters. Perfusion increase in all arterial territories on both ipsilateral and contralateral hemispheres was noted 1 day after CEA by Rijbroek et al. [4].
Non-radionuclide techniques such as functional MRI [16] or PCT [1] also demonstrated improvement of cerebral hemodynamics after revascularization procedures.
We did not measure absolute values of cerebral perfusion; our evaluation is based on relative radiotracer distribution in the brain. Interhemispheric asymmetry was previously found to be useful for image assessment before and after revascularization procedures. Sfyroeras et al. [12] demonstrated with 99mTc-HMPAO SPECT wide variation of AI before carotid stenting. Immediately after treatment, AI improved (although without statistical significance). Wilson et al. [17] suggested association between cerebral blood flow asymmetry on post-CEA magnetic resonance perfusion brain scans and cognitive dysfunction; however, the study population was rather small (n=22) and authors call for continued investigations. Waaijer et al. [1] concluded that relative CT perfusion values based on interhemispheric comparison are better suited (compared with absolute perfusion CT values) for demonstrating changes in cerebral perfusion after CEA or stent placement in patients with unilateral symptomatic carotid artery stenosis. Goode et al. [16] found that patients with abnormal preoperative asymmetry of cerebrovascular reserve showed greater hemodynamic improvement following CEA, based on pre-and post-CEA functional MRI study of 17 patients with symptomatic artery stenosis.
The most relevant and novel finding of our study is the relationship between preoperative interhemispheric perfusion asymmetry and percentage increase of perfusion in the ipsilateral hemisphere after CEA. To the best of our knowledge, direct correlation between these parameters has not been reported before. In our opinion, AI assessment may be useful in clinical practice. It can be easily and quickly obtained from brain perfusion SPECT, which is a noninvasive diagnostic tool that is widely available and relatively inexpensive. On one hand, in patients with high preoperative AI we can expect greater perfusion improvement after revascularization, which is prognostically favorable. On the other hand, rapid reperfusion may be dangerous to the patient. A severe and diffuse asymmetric pattern of preoperative perfusion is considered to be one of the factors predictive of cerebral hyperperfusion syndrome after CEA or carotid stenting. These factors were determined by SPECT studies [18,19]. Apart from AI, they include patient age and impaired cerebrovascular reactivity. Hyperperfusion, defined as a perfusion increase of ≥100%, is a rare but disastrous complication after revascularization, therefore extreme procedural care is postulated in patients at risk [19].
For semiquantitative evaluation of perfusion, most studies have used region of interest approaches. These methods are operator-dependent, time-consuming, and the analysis does not include the entire brain. The newer image processing techniques such as voxel-based analysis applied in the present work are automatic, 3-dimensional, and give fast, objective assessment of the whole brain. Comparison of baseline and control SPECT without dedicated software is especially difficult, even for an experienced specialist in nuclear medicine, whereas the voxel-based approach guarantees accuracy of obtained results.
Assessment of vasodilatory reserve with acetazolamide is often a part of brain perfusion imaging in the context of cerebrovascular disease. Lack of this test is a limitation of the present study. Reduced vascular reserve carries a higher risk for hyperperfusion after CEA [18] and predicts development of cerebral ischemic lesions caused by microemboli during the procedure [6]. Moreover, in patients with morphological lesions detected by CT, hypoperfused regions without improvement after CEA may result from diaschisis. This phenomenon accompanies lesions of various etiologies, including infarction [20]. The injury interrupts connections with other cerebral regions, which receive weak afferent signals and subsequently decrease their metabolism and perfusion [21]. Without assessment of cerebrovascular reserve, we cannot differentiate between diaschisis and hemodynamic impairment. The second limitation is evaluation of perfusion based on relative radiotracer distribution in the brain, instead of absolute values. However, our study was designed to explore association between preoperative perfusion pattern and reperfusion after CEA by means of a widely available, non-invasive examination that can be useful in daily clinical practice, whereas both acetazolamide test and absolute cerebral blood flow measurement are complicated procedures. The former gives the possibility of adverse effects [22], and increases radiation burden and patient inconvenience.
Conclusions
Brain perfusion SPECT with voxel-based analysis allows for detection of hypoperfusion in patients with ICA stenosis, including asymptomatic and symptomatic, without morphological changes in CT. Our results suggest that the perfusion increase detected in the majority of examined patients 3–5 days after CEA is higher in cases with greater ipsilateral asymmetry index. Thus, evaluation of preoperative AI may help to identify patients in whom rapid reperfusion is more likely.
Footnotes
Source of support: Departmental sources
References
- 1.Waaijer A, van Leeuwen MS, van Osch MJ, et al. Changes in cerebral perfusion after revascularization of symptomatic carotid artery stenosis: CT measurement. Radiology. 2007;245:541–48. doi: 10.1148/radiol.2451061493. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda T, Ogasawara K, Kobayashi M, et al. Prediction of cerebral hyperperfusion after carotid endarterectomy using cerebral blood volume measured by perfusion-weighted MR imaging compared with single-photon emission CT. AJNR Am J Neuroradiol. 2007;28:737–42. [PMC free article] [PubMed] [Google Scholar]
- 3.Hino A, Tenjin H, Horikawa Y, et al. Hemodynamic and metabolic changes after carotid endarterectomy in patients with high-degree carotid artery stenosis. J Stroke Cerebrovasc Dis. 2005;14:234–38. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Rijbroek A, Boellaard R, Vermeulen EG, et al. Hemodynamic changes in ipsi- and contralateral cerebral arterial territories after carotid endarterectomy using positron emission tomography. Surg Neurol. 2009;71:668–76. doi: 10.1016/j.surneu.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Ogasawara K, Kobayashi M, Suga Y, et al. Significance of postoperative crossed cerebellar hypoperfusion in patients with cerebral hyperperfusion following carotid endarterectomy: SPECT study. Eur J Nucl Med Mol Imaging. 2008;35:146–52. doi: 10.1007/s00259-007-0588-x. [DOI] [PubMed] [Google Scholar]
- 6.Aso K, Ogasawara K, Sasaki M, et al. Preoperative cerebrovascular reactivity to acetazolamide measured by brain perfusion SPECT predicts development of cerebral ischemic lesions caused by microemboli during carotid endarterectomy. Eur J Nucl Med Mol Imaging. 2009;36:294–301. doi: 10.1007/s00259-008-0886-y. [DOI] [PubMed] [Google Scholar]
- 7.Chida K, Ogasawara K, Aso K, et al. Postcarotid Endarterectomy Improvement in Cognition Is Associated with Resolution of Crossed Cerebellar Hypoperfusion and Increase in I-Iomazenil Uptake in the Cerebral Cortex: A SPECT Study. Cerebrovasc Dis. 2010;29:343–51. doi: 10.1159/000278930. [DOI] [PubMed] [Google Scholar]
- 8.Tawes RL, Lull R. Value of single photon emission computerized imaging in the treatment of patients undergoing carotid endarterectomy. J Vasc Surg. 1996;24:219–25. doi: 10.1016/s0741-5214(96)70097-6. [DOI] [PubMed] [Google Scholar]
- 9.Engelhardt M, Pfadenhauer K, Zentner J, et al. Impaired cerebral autoregulation in asymptomatic patients with carotid artery stenosis: comparison of acetazolamide-SPECT and transcranial CO(2)-dopplersonography. Zentralbl Chir. 2004;129:178–82. doi: 10.1055/s-2004-822798. [DOI] [PubMed] [Google Scholar]
- 10.Chaves C, Hreib K, Allam G, et al. Patterns of cerebral perfusion in patients with asymptomatic internal carotid artery disease. Cerebrovasc Dis. 2006;22:396–401. doi: 10.1159/000094858. [DOI] [PubMed] [Google Scholar]
- 11.Chaves CJ, Staroselskaya I, Linfante I, et al. Patterns of perfusion-weighted imaging in patients with carotid artery occlusive disease. Arch Neurol. 2003;60:237–42. doi: 10.1001/archneur.60.2.237. [DOI] [PubMed] [Google Scholar]
- 12.Sfyroeras GS, Arsos G, Karkos CD, et al. Interhemispheric asymmetry in brain perfusion before and after carotid stenting: a 99mTc-HMPAO SPECT study. J Endovasc Ther. 2006;13:729–37. doi: 10.1583/06-1857.1. [DOI] [PubMed] [Google Scholar]
- 13.Vanninen E, Kuikka JT, Aikiä M, et al. Heterogeneity of cerebral blood flow in symptomatic patients undergoing carotid endarterectomy. Nucl Med Commun. 2003;24:893–900. doi: 10.1097/01.mnm.0000084578.51410.20. [DOI] [PubMed] [Google Scholar]
- 14.Lishmanov Y, Shvera I, Ussov W, Shipulin V. The effect of carotid endarterectomy on cerebral blood flow and cerebral blood volume studied by SPECT. J Neuroradiol. 1997;24:155–62. [PubMed] [Google Scholar]
- 15.Otte A, Ostwald E, Rem JA, et al. Effect of thrombus endarterectomy (TEA) on the regional cerebral bloodflow (rCBF) in patients with unilateral internal carotid artery stenosis. Nuklearmedizin. 1997;36:23–28. [PubMed] [Google Scholar]
- 16.Goode SD, Altaf N, Auer DP, Macsweeney ST. Carotid Endarterectomy Improves Cerebrovascular Reserve Capacity Preferentially in Patients with Preoperative Impairment as Indicated by Asymmetric BOLD Response to Hypercapnia. Eur J Vasc Endovasc Surg. 2009;38:546–51. doi: 10.1016/j.ejvs.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Wilson DA, Mocco J, D’Ambrosio AL, et al. Post-carotid endarterectomy neurocognitive decline is associated with cerebral blood flow asymmetry on post-operative magnetic resonance perfusion brain scans. Neurol Res. 2008;30:302–6. doi: 10.1179/016164107X230540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosoda K, Kawaguchi T, Ishii K, et al. Prediction of hyperperfusion after carotid endarterectomy by brain SPECT analysis with semiquantitative statistical mapping method. Stroke. 2003;34:1187–93. doi: 10.1161/01.STR.0000068781.31429.BE. [DOI] [PubMed] [Google Scholar]
- 19.Kaku Y, Yoshimura S, Kokuzawa J. Factors predictive of cerebral hyperperfusion after carotid angioplasty and stent placement. AJNR Am J Neuroradiol. 2004;25:1403–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Flores LG, II, Futami S, Hoshi H, et al. Crossed cerebellar diaschisis: analysis of iodine-123-IMP SPECT imaging. J Nucl Med. 1995;36:399–402. [PubMed] [Google Scholar]
- 21.Catafau AM. Brain SPECT in clinical practice. Part I: perfusion. J Nucl Med. 2001;42:259–71. [PubMed] [Google Scholar]
- 22.Derick RJ. Carbonic anhydrase inhibitors. In: Mauger TF, Craig EL, editors. Hevener’s Ocular Pharmacology. 6th ed. St. Louis: C.V. Mosby Co; 1994. pp. 78–99. [Google Scholar]


