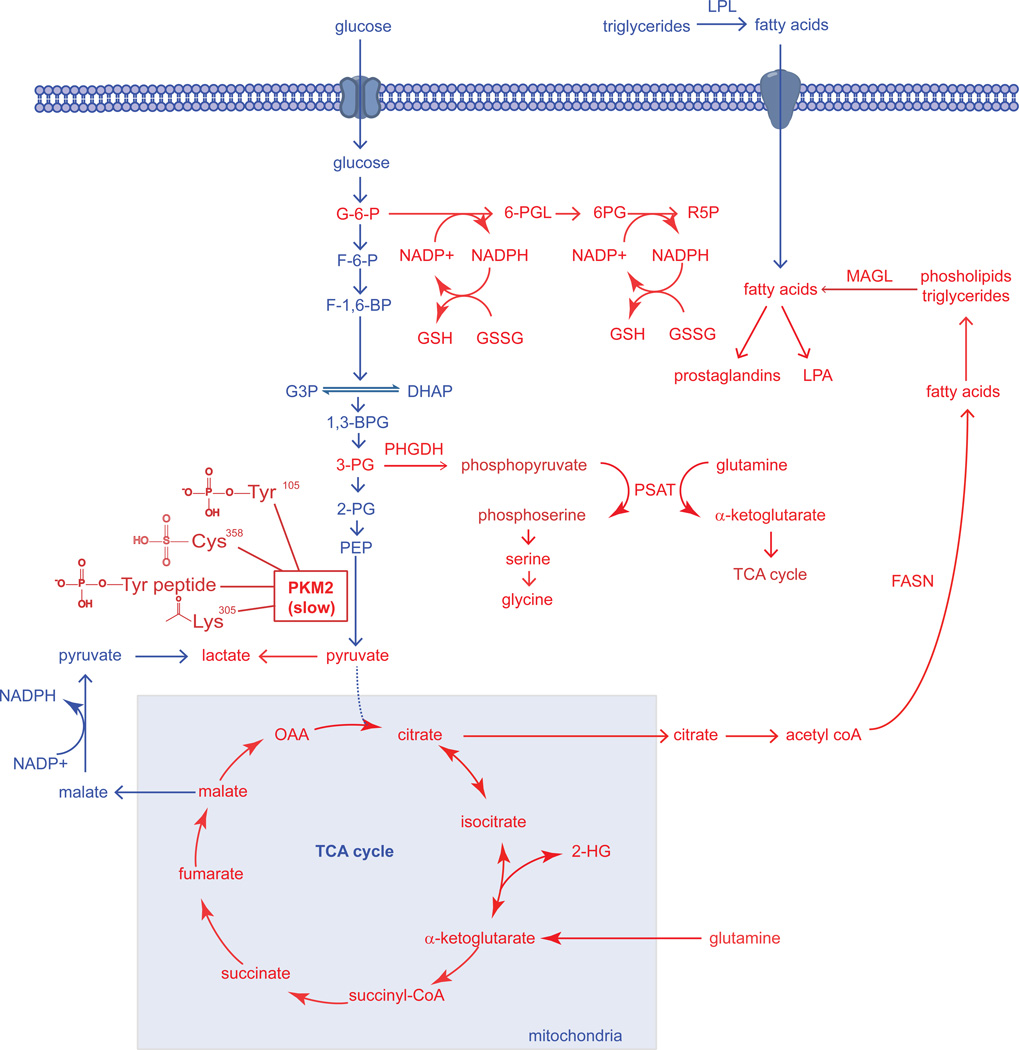
Figure 1. Dysregulated Metabolic Pathways in Cancer Cells Identified Thus Far.
The metabolic pathways that sustain the proliferative nature of a cancer cells are very much the same pathways that constitute the metabolism of normal cells. However, cancer cells are able to aberrantly rewire many of these normal pathways to meet their excessive needs for growth and proliferation. In the figure above, we see that pathways that have been revealed to be essential in cancer cells (shown in Red) are pathways that are fundamentally important for the synthesis of biological macromolecules, antioxidants, and signaling molecules that facilitate cellular growth, survival, and progression. The “Warburg effect,” the preferential shunting of pyruvate to lactate under conditions of normoxia, is facilitated by the switch from the M1 splice isoform of pyruvate kinase to the M2 splice isoform, which is in-general less active due to several modes of regulation on PKM2 activity in the form of protein-protein interactions or posttranslational modifications which all collectively inhibit PKM2 activity (Anastasiou et al., 2011; Christofk et al., 2008b; Hitosugi et al., 2009; Lv et al., 2011). The slow nature of this M2 splice isoform allows for the buildup of upstream glycolytic intermediates rather than favoring the flux of glycolytic carbon through the TCA cycle. Two of the important upstream glycolytic intermediates that are essential for synthesizing necessary biological macromolecules are glucose-6-phosphate (G6P) and 3-phosphoglycerate (3PG). The 3-step formation of Ribose-5-phosphate (R5P) via 6-phosphogluconolactone (6-PGL) and 6-phosphogluconate (6-PG), involves the production of reducing equivalent in the form of NADPH, which in-turn can be used as the primary reducing power in the production of glutathione (GSH) from its oxidized precursor glutathione disulfide (GSSG) (Anastasiou et al., 2011). It was also discovered that a significant portion of glycolytic carbon is diverted towards serine and glycine biosynthesis by phoshphoglycerate dehydrogenase (PHGDH), which catalyzes the conversion of 3-PG to phosphopyruvate. Phosphopyruvate is then converted to phosphoserine, concurrently with the anaplerotic generation of α-ketoglutarate by glutamine to supply the TCA cycle (Locasale et al., 2011a; Possemato et al., 2011). Glutaminolysis-derived α-ketoglutarate provides reducing power in the form of NAPDH and regenerates oxaloacetate (OAA) to form citrate which are both necessary for the production of fatty acids by fatty acid synthase (FASN) (DeBerardinis et al., 2007; Metallo et al., 2012). Under hypoxia or mitochondrial dysfunction, α-ketoglutarate can also undergo reductive carboxylation to isocitrate and then to citrate to support lipogenesis (Mullen et al., 2012). Upon hypoxia or if IDH1 or IDH2 is mutated, these enzymes can also form the oncometabolite 2-hydroxyglutarate (2-HG) (Dang et al., 2010; Wise et al., 2011). Upon synthesis of fatty acids and esterification onto phospholipids or triglycerides, these esterified lipids are mobilized through lipolytic processes involving enzymes such as monoacylglycerol lipase (MAGL), which can lead to the formation of oncogenic lipid signaling molecules including lysophosphatidic acid (LPA) and prostaglandins (Nomura et al., 2010b). Fatty acids can also arise from extracellular sources by hydrolysis of triglycerides by lipoprotein lipase (LPL).