Figure 2.
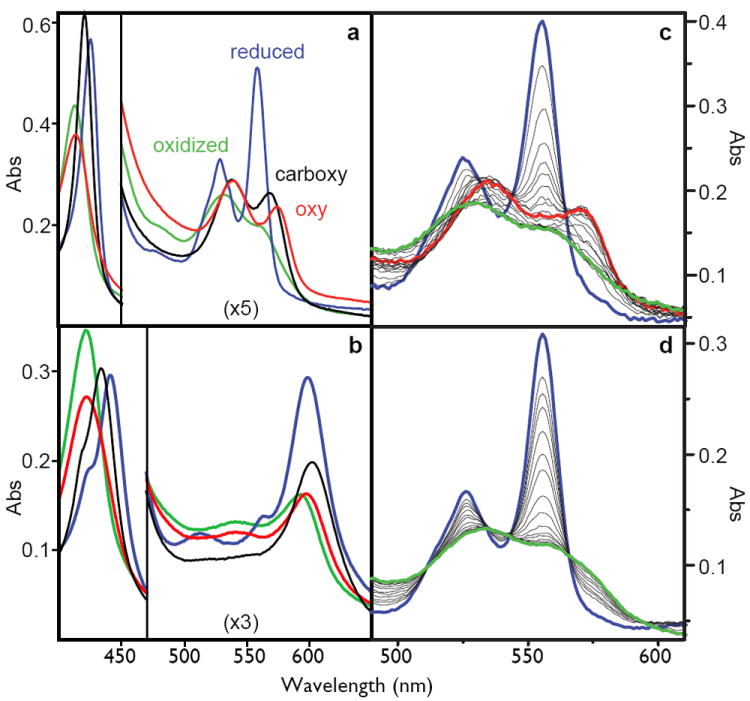
Left: the spectra of the oxidized (green), reduced (blue), carboxy-ferrous (black) or oxy-ferrous (red) artificial oxygen transport protein 6 with either heme B (A) or heme A as the cofactor (B). These spectra are obtained at -15C where these spectra are stable for more than an hour. Right: stopped-flow spectral changes for mixing the reduced heme B proteins with oxygen at 15C. The fully designed oxygen transport protein 6 (C), shows the transformation of the reduced heme (blue) to the oxy-ferrous state (red) which eventually becomes oxidized (green), while the early intermediate 2 (D) proceeds directly and rapidly to the oxidized form.
