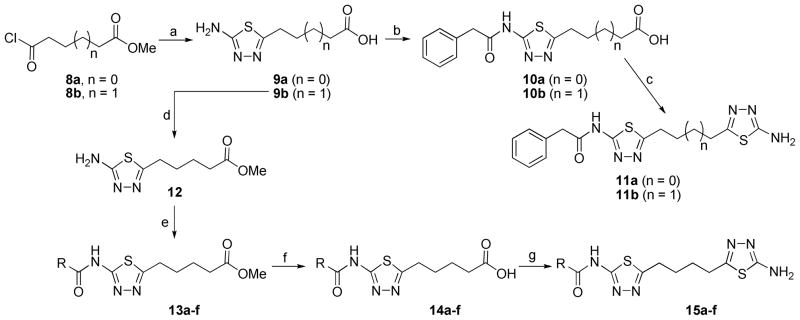
Scheme 2.
Synthesis of 11a–b and 15a–fa
a Reagents and conditions: (a) thiosemicarbazide, POCl3, 85 °C, 72% for 9a and 89% for 9b; (b) phenylacetyl chloride, TEA, THF, 15% for 10a and 92% for 10b; (c) thiosemicarbazide, POCl3, 85 °C, 4% for 11a and 45% for 11b; (d) H2SO4, MeOH, 65 °C, 71%; (e) acid chloride, TEA, DCM, 48–95%; (f) LiOH, MeOH, H2O, 40 °C, 63–91%; (g) thiosemicarbazide, POCl3, 85 °C, 18–57%.