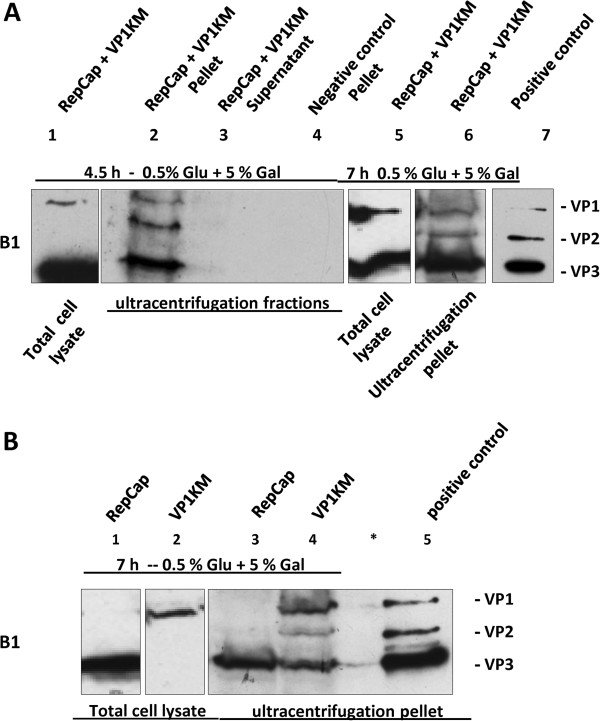
Figure 6.
Concentration of AAV2 capsid like-structures by high-speed ultracentrifugation through 40% sucrose-cushion. (A): YEplacRepCap + pYESVP1KM (RepCap + VP1KM)co-transformed yeast cells induced for 4.5 h (lanes 1–4) or 7 h (lanes 5 and 6) in 0.5% Glu + 5% Gal medium were subjected to a small scale (2 x 108 cells) - protein extraction yielding total cell lysate (lanes 1 and 5) and to a large scale extraction (~400 x 108 cells) (lanes 2, 3, 4 and 6) under non-denaturing conditions yielding the “native extract“ which was subjected to ultracentrifugation. As indicated on the bottom, the total cell lysate (lanes 1 and 5) and the resulting ultracentrifugation fractions, the supernatant (lane 3) and the pellet (lanes 2 and 6), were analyzed for the presence of VP proteins by Western blot. The antibody mAb B1 recognized only VP1 ad VP3 in the total cell lysate and all three VPs in the pellet fraction. The VP ratios in the total cell lysate were 1:8 (lane 1) and 1:3.3 (lane 5). The VP ratios in the pellet resulting from ultracentrifugation were 1:1.2:6.5 (lane 2), 1:3 (lane 6). The pellet obtained by ultracentrifugation of the extracts derived from cells co-transformed with empty vectors, YEplac181 and pYES2, was used as negative control (lane 4). Positive control is loaded in lane 7. (B): Cells transformed with YEplacRepCap (RepCap, lane 1 and 3) or with pYESVP1KM (VP1KM, lane 2 and 4) were induced for 7 h in 0.5% glucose + 5% galactose medium. All cells were processed as described in (A). VP proteins in the total cell lysates (lanes 1 and 2) and the ultracentrifugation pellet (lanes 3 and 4) were identified by Western blot analysis with mAb B1. The relative VP ratio is 1:0.25:0.9 for sample loaded in lane 4. No proteins were loaded in lanes marked with *. In the lane 5 the positive control was loaded.