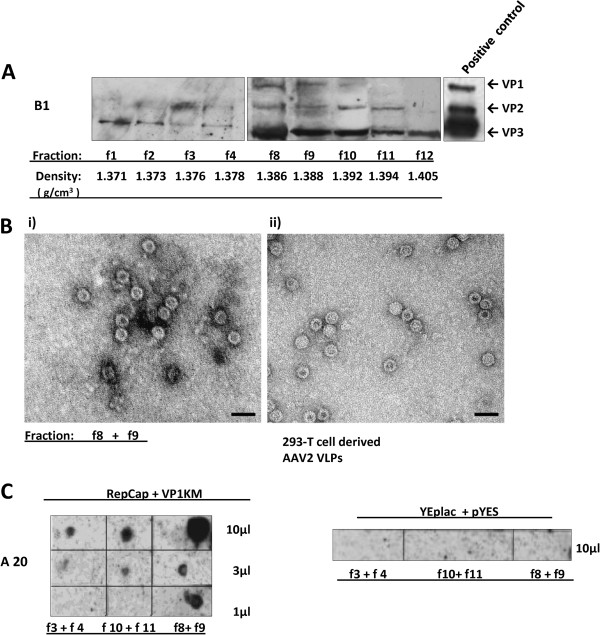
Figure 7.
Isolation of AAV2 capsid like-structures by ultracentrifugation in CsCl-gradient. Native protein extracts derived from ~ 0.5x1012 YEplacRepCap + pYESVP1KM (RepCap + VP1KM)co-transformed yeast cells, induced under optimal conditions, were subjected to 40% sucrose cushion-ultracentrifugation and the pelleted material was further fractionated in CsCl gradient by 48 h,. (A): 12 CsCl fractions of increasing densities were recovered and analyzed for the presence of VP proteins by Western blot with mAb B1. Only VP positive fractions are presented. Structures recovered in fractions 8–11 had VP compositions that most closely resembled the one of wt capsids. Denatured 293 T-cell derived AAV2 capsids were used as positive control for defining VPs. (B): Fractions of similar densities were united and subjected to TEM analysis. (i) Capsid-like structures of ~20 nm size identified in fraction f8 + f9 are shown and compared with 293 T –derived AAV2 empty capsids (ii). Scale bar is 40 nm. (C): 3 fraction pairs that gave positive results in TEM were spotted on the nitrocellulose membrane in three quantities indicated on the right side bar and analyzed for the presence of AAV capsids with the capsid-specific mAb A20 antibody. The strongest signal (which indicates the greatest number of capsids) was detected in the fraction f8 + f9. As negative control of the assay, the same number of cells co-transformed with empty vectors, YEplac181 and pYES2, were processed as described in (A) and the obtained CsCl fractions of the corresponding densities were incubated with A20 antibody. The name of fractions and relative density are indicated.