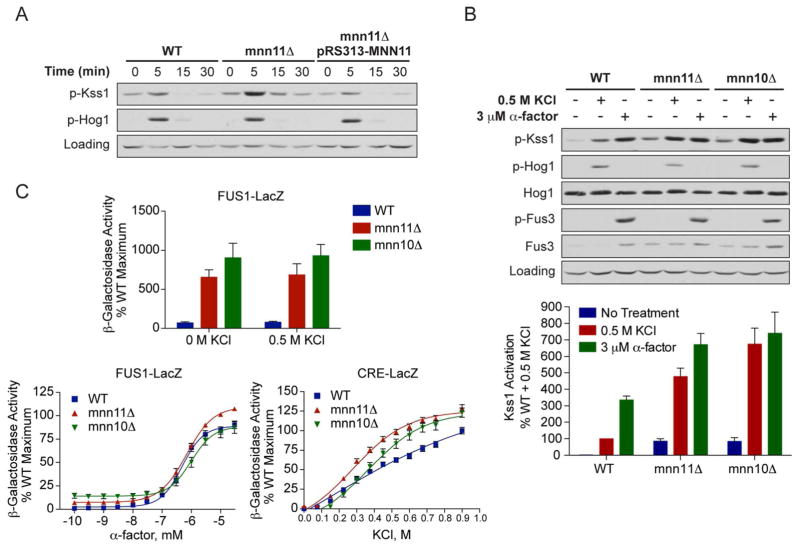
Figure 2. Deletion of MNN11 results in hyperactivation of Kss1 and the filamentous growth pathway.
A) Activation of Kss1 and Hog1; Wild-type, mnn11Δ and pRS313-MNN11-transformed mnn11Δ cells were stimulated with 0.5 M KCl for the indicated times. Cell lysates were resolved by 10% SDS-PAGE. Phospho-Kss1 (p-Kss1) and phospho-Hog1 (p-Hog1) were detected by immunoblotting with phospho-p44/42 and phospho-p38 antibodies, which recognize the dually phosphorylated and activated forms of Kss1 and Hog1, respectively. G6PDH served as a loading control. (B) Activation of Kss1, Hog1 and Fus3; WT, mnn10Δ and mnn11Δ cells were stimulated with 0.5 M KCl for 5 min or with 3 μM α-factor pheromone for 30 min. Cell lysates were resolved by 10% SDS-PAGE. Specific antibodies were used to detect the activated form of Hog1, Kss1 and Fus3 (p-Fus3). Total Hog1 and Fus3 abundance were determined with Hog1 and Fus3 antibodies. G6PDH served as a loading control. All primary antibodies were recognized by chemiluminescent detection and quantified by scanning densitometry (ImageJ). The bottom panel shows averaged scanning densitometry data for three individual experiments. Error bars represent ± SEM. (C) Transcriptional activation (β-galactosidase activity) was measured spectrofluorometrically in wild-type (WT), mnn10Δ and mnn11Δ cells transformed with a plasmid containing either a pheromone-inducible reporter (FUS1-lacZ) or a salt-inducible reporter (CRE-lacZ). Transcription was induced by the addition of the indicated concentrations of KCl or α-factor pheromone. Data are the mean ± SE of four individual colonies measured in triplicate and presented as a percentage of wild-type maximum.