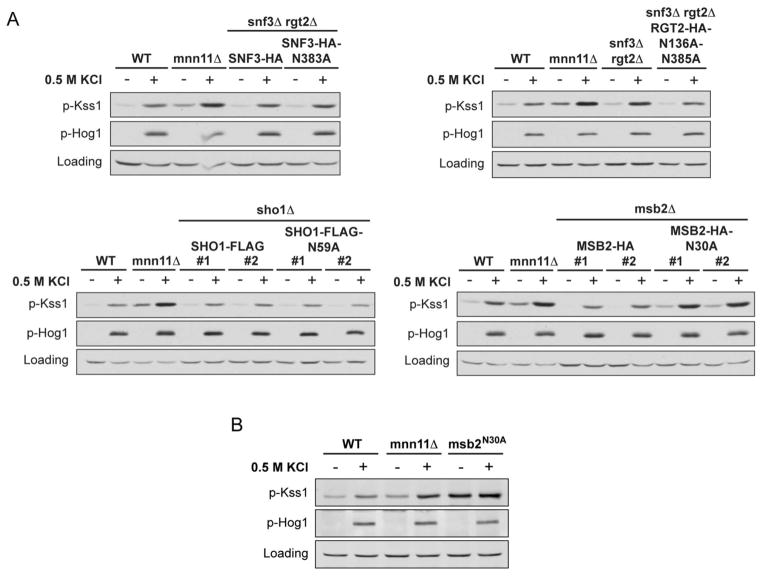
Figure 6. Msb2 Asn-30 is a putative N-glycosylation site required to limit the activation of Kss1 by osmotic stress.
(A) Activation of Kss1 and Hog1; mnn11Δ, snf3Δrgt2Δ, sho1Δ, and msb2Δ mutant strains, transformed with pRS316-SNF3-HA, pRS313-RGT2-HA, pRS315-SHO1-FLAG, pRS316-MSB2-HA, or the indicated glycosylation-site mutants, were stimulated with 0.5 M KCl for 5 min. Cell lysates were resolved by 10% SDS-PAGE and immunoblotting. Specific antibodies were used to detect the dually phosphorylated, fully activated forms of Kss1 and Hog1 (p-Kss1 and p-Hog1). G6PDH served as a loading control. For the relevant uncomplemented deletion strains, refer to Figures 4 and 5. (B) Activation of Kss1 and Hog1 in wild-type, mnn11Δ or integrated msb2N30A mutant, stimulated with 0.5 M KCl for 5 min (69).