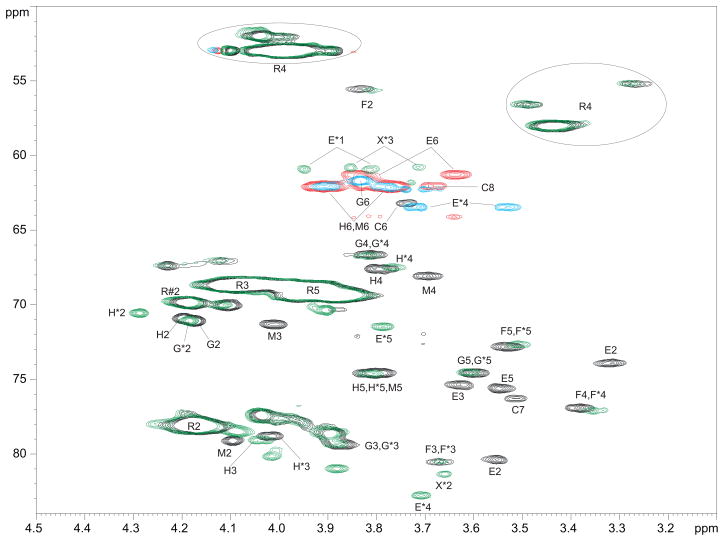
Fig. 2.
Fragment of 1H-13C HSQC NMR spectra of B. suis polysaccharide (CH - black, CH2 - red) and NaIO4 oxidized, NaBD4-reduced, AcOH hydrolyzed B. suis polysaccharide (CH - green, CH2 - blue). Oxidized product signals are marked with *; R is 2- or 3-substituted Rha4NFo; R#2 is the signal of H/C-2 of 3-substituted Rha4NFo. Note the disappearance of units C, E, and M signals after the oxidation. Signals of mannose H shift probably due to the removal of the residue M, which was close to H. Hydroxymethyl groups E*1 and X*3 appear as CH because of deuterium substitution. R4 appears at ~3.4 and 4.0 ppm due to formyl group E/Z tautomerism.