Figure 1.
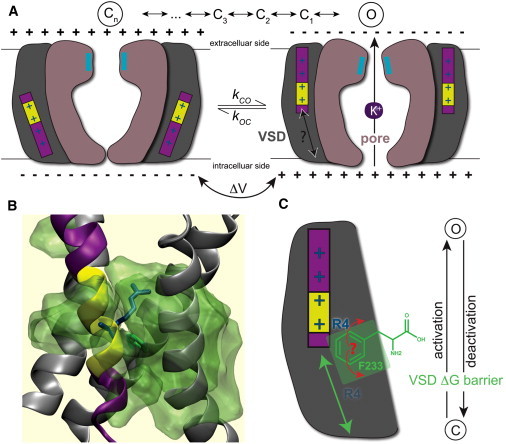
How different is activation from deactivation for the VSD S4 translation? (A) The translation of the charged S4 helix leads to a conformational change in the pore domain. The (de)activation comprises several intermediate states corresponding to S4 charges crossing a hydrophobic core, likely aided by a local 310-helix (yellow) segment sliding along the sequence as S4 translates. (B) Simulation system (white lipid chains, blue/red head-groups, and blue water) with the central hydrophobic core of the VSD is highlighted in transparent green. The R4 (R299) gating charge and F233 lock are drawn in blue, green sticks, respectively. (C) The phenyl ring has to rotate for the gating charges to cross, but is it merely pushed away in the direction of S4 translation, or can it help explain differences between activation and deactivation?
