Figure 2.
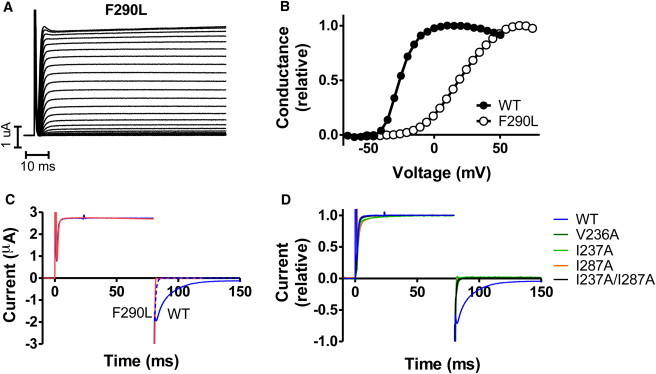
The F233L mutation destabilizes the open state. (A) Representative current family of the Kv1.2/2.1 F233L mutation (Shaker F290L) in control solution. The holding voltage was set to −80 mV and test pulses ranging from −80 to +75 mV in 5 mV increments. (B) Representative curves for WT and F233L (Shaker F290L). The F233L mutation shifts the channel voltage dependence toward more positive voltages. (C) Representative currents for channel activation (stepping to +50 mV from a holding voltage of −80 mV) and deactivation (stepping to −50 mV from an activation voltage of +50 mV) for WT (blue), and Shaker F290L (red). Legend residue numbers refer to Shaker. Experiments were performed in a high-potassium solution (see Methods). The activation is not affected by the F233L mutation, although channel deactivation is speeded up dramatically. The dashed blue curve is WT closure speeded up by a factor of 10. F290L is clearly faster. Panel D displays the same condition as in C but relative currents for mutations in the hydrophobic ring surrounding Shaker F290 (Kv1.2/2.1 F233).
