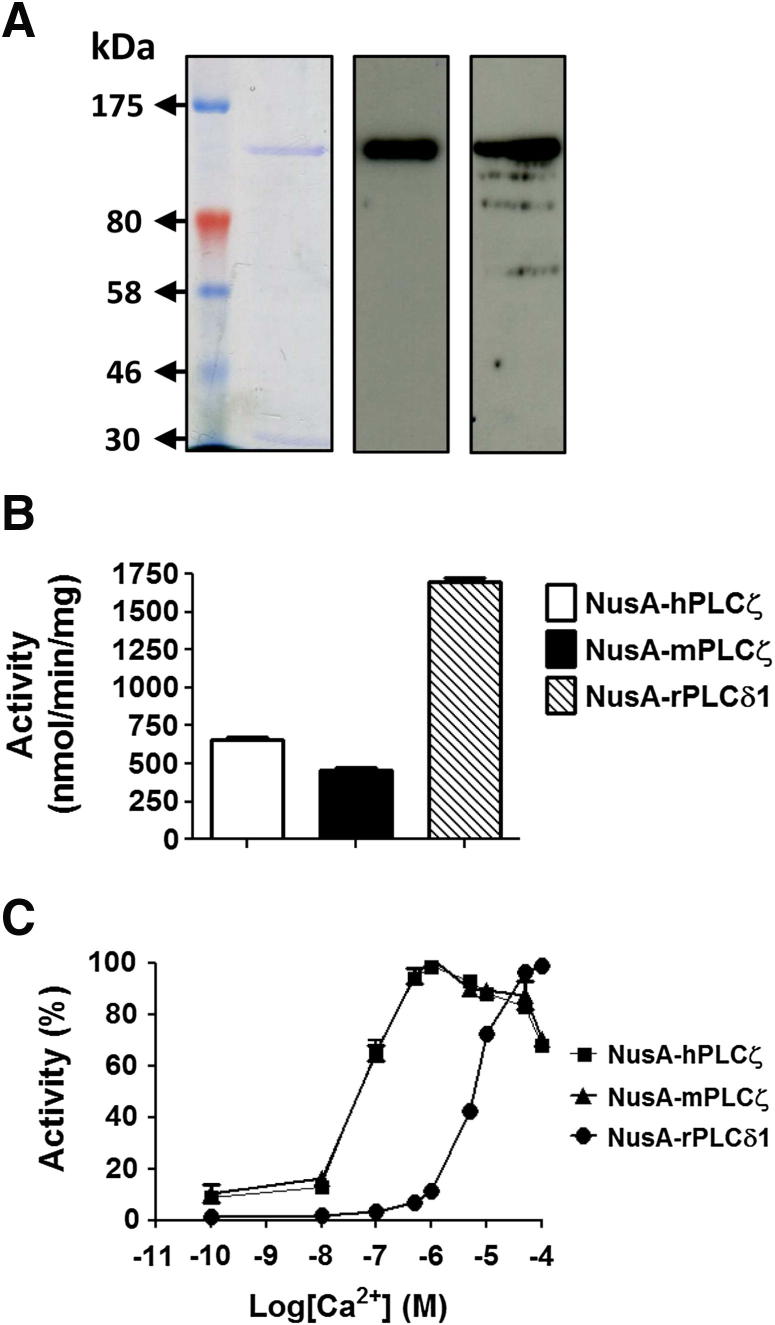
Figure 2.
Expression and enzymatic characterization of recombinant wild-type human phospholipase Cζ (PLCζ) protein. (A) One μg of bacterially-expressed, affinity-purified NusA-hPLCζ fusion protein analyzed by 7% SDS-PAGE (left panel) or by immunoblot analysis with either anti-PLCζ polyclonal (V-37; 1:10,000 dilution; middle panel) or anti-NusA monoclonal antibody (1:20,000 dilution; right panel). (B) The PIP2 hydrolysis enzyme activities of recombinant hPLCζ, mPLCζ, and rPLCδ1 purified by nickel affinity chromatography as NusA-fusion proteins (20 pmol) determined with the [3H]PIP2 cleavage assay, n = 3 ± standard error of the mean (SEM), using two different preparations of recombinant protein and with each experiment performed in duplicate. In control experiments with NusA alone, no specific PIP2 hydrolysis activity was observed (data not shown). (C) Effect of varying [Ca2+] on the normalized PIP2 hydrolysis enzyme activity of purified, recombinant hPLCζ, mPLCζ, and rPLCδ1 NusA-fusion proteins. For these assays, n = 2 ± SEM using two different batches of recombinant proteins and with each experiment performed in duplicate.