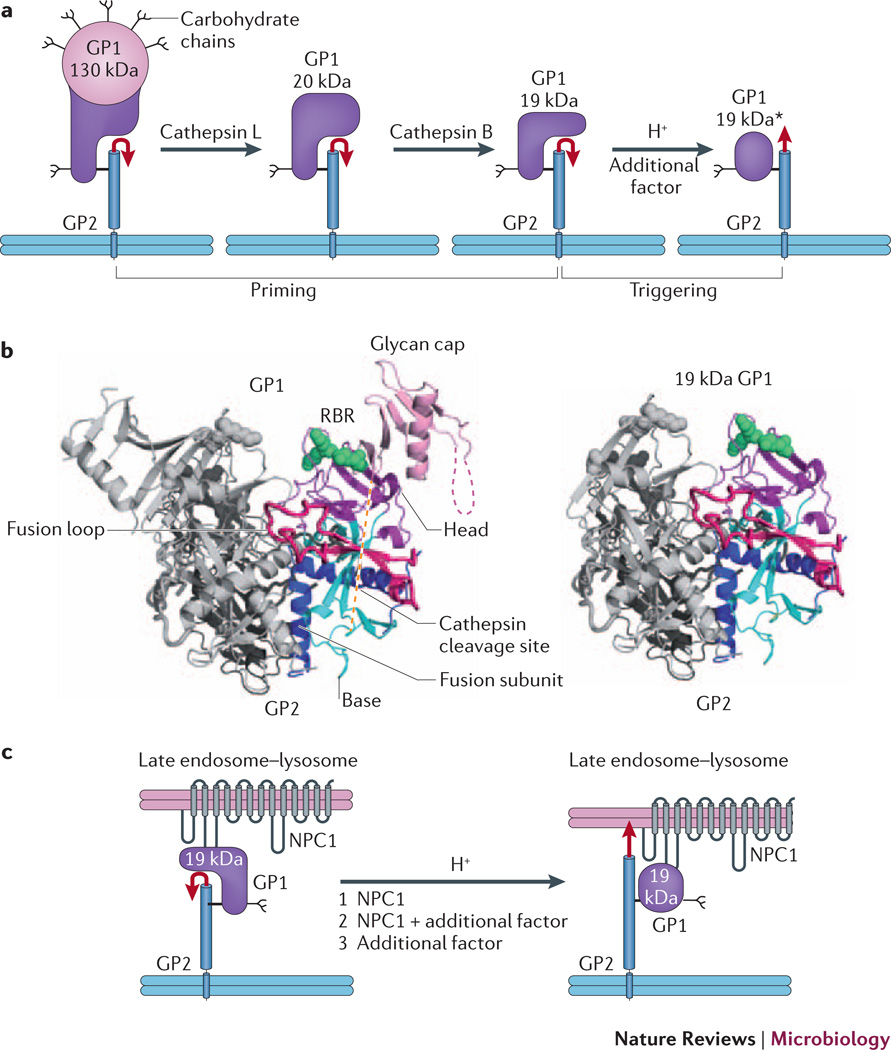
Figure 2. EBOV GP priming and triggering: possible roles of NPC1.
a | Cathepsin L and cathepsin B cleave EBOV GP1, removing the glycan cap and mucin-like domain (pink), thereby generating a 20 kDa and then a 19 kDa form of the glycoprotein. Primed 19 kDa GP requires low pH and the activity of an additional factor to trigger rearrangements that liberate and relocate the fusion loop (red arrow), and thereby initiate the fusion cascade. b | Ribbon diagram of GPΔ (left, PDB 3CSY) and a model for 19 kDa GP (right). The model for cathepsin-primed GP (right), assumes that no conformational changes occur upon cathepsin cleavage. One monomer of each trimer is colored and the other two are in grey. Green spheres depict Lys114, Lys115 and Lys140 (in the RBR), which when mutated impair primed GP binding to NPC1 and GP-mediated infection. c | Possible roles for NPC1 in triggering EBOV fusion. Following delivery to a late endosome–lysosome and priming by cathepsins, primed GP binds to the C-loop of NPC1. In a first model (1), NPC1 is the additional factor depicted in part a that, together with low endosomal pH, triggers the conformational changes that liberate and relocate the fusion loop (red arrow), leading to fusion and therefore penetration of the genome into the cytoplasm to initiate replication. In a second model (2), NPC1 binding to primed GP is necessary, but not sufficient, to trigger GP, and one or more additional factors are still required. In a third scenario (3), NPC1 binds to primed GP, linking the virus to the endosomal membrane, but does not induce conformational changes in GP; in this case an additional factor triggers fusion. If model 3 is correct, NPC1 must have important roles closely upstream of fusion triggering; it may also have an additional upstream role (or roles) if model 1 or 2 applies. Part a is modified with permission from reference30.