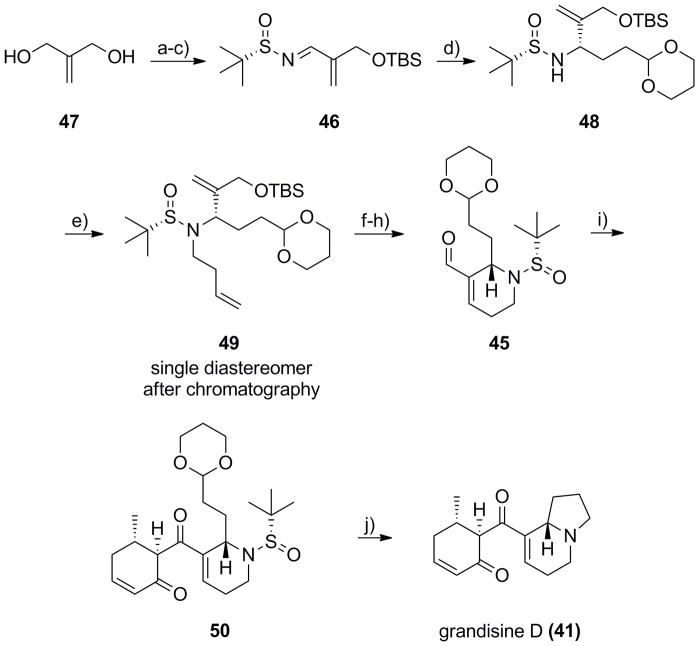
Scheme 8.
Synthesis of (+)-grandisine D (41). a) TBSCl, imidazole, CH2Cl2,95%; b) MnO2, CH2Cl2, 90%; c) (S)-tert-butyl sulfinamide, Ti(OEt)4, CH2Cl2, 87%; d) (2(-1,3- dioxan-2-yl)ethyl)magnesium bromide, THF, −78 °C to −45 °C, 79% (>10:1 dr); e) LiHMDS, 3-buteynyl triflate, HMPA:THF, −78 °C, 87%; f) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 96%; g) TBAF, THF, 0 °C, 93%; h) MnO2, CH2Cl2, 100%; i) i) 44, n-Bu2BOTf, i-PrNEt2, CH2Cl2, −78 °C to rt, ii) TFAA, DMSO, CH2Cl2, −78 °C, 77%; j) i) TFA:H20 (95:5), rt, 45 min; ii) PS-BH(OAc)3, DCE, 47%.