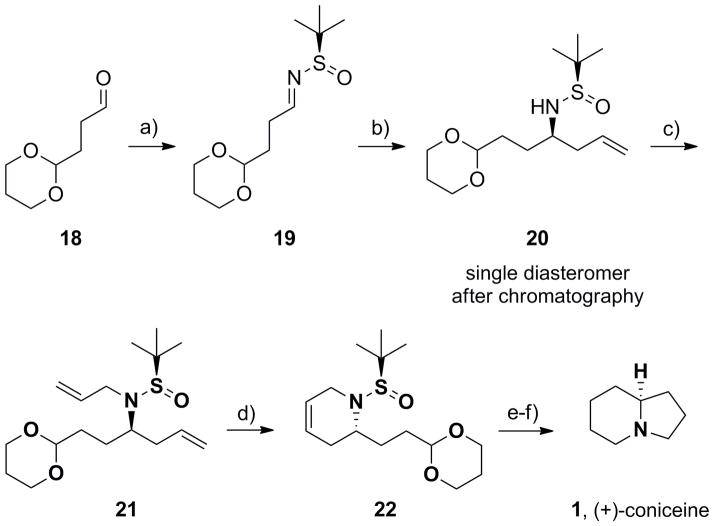
Scheme 3.
Synthesis of (−)-coniceine (1), representing the basic indolizidine core. a) (R)-tert-butyl sulfinamide, CuSO4, CH2Cl2, 91%; b) In(0), allyl bromide, sat’d aq. NaBr, rt, 16 h, 87% (>15:1 dr); c) LiHMDS, allyl bromide, DMF, −20 °C to rt, 80%; d) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 84%; e) H2, Pd/C; f) i) TFA:H20 (95:5), rt, 45 min; ii) PS-BH(OAc)3, 81% for two steps.