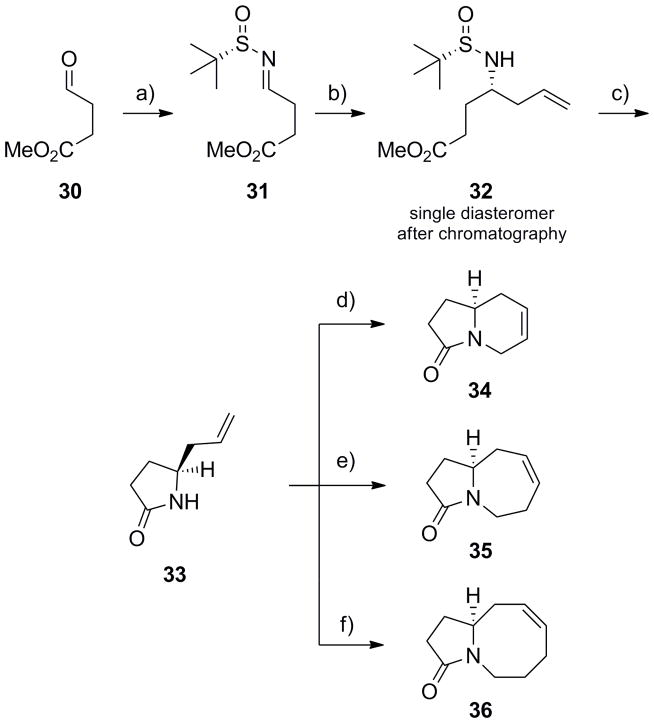
Scheme 6.
Synthesis of azabicyclic lactams 37–39. a) (S)-tert-butyl sulfinamide, CuSO4, CH2Cl2, 90%; b) In(0), allyl bromide, sat’d aq. NaBr, rt, 16h, 88% (>10:1 dr); c) i) HCl, MeOH, rt, ii) Na2CO3, CH2Cl2, 97%; d) i) LiHMDS, allyl bromide, DMF, −20 °C to rt; ii) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 87% for two steps; e) i) LiHMDS, butenyl bromide, DMF, −20 °C to rt; ii) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 86% for two steps; f) i) LiHMDS, pentenyl bromide, DMF, −20 °C to rt; ii) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 73% for two steps.