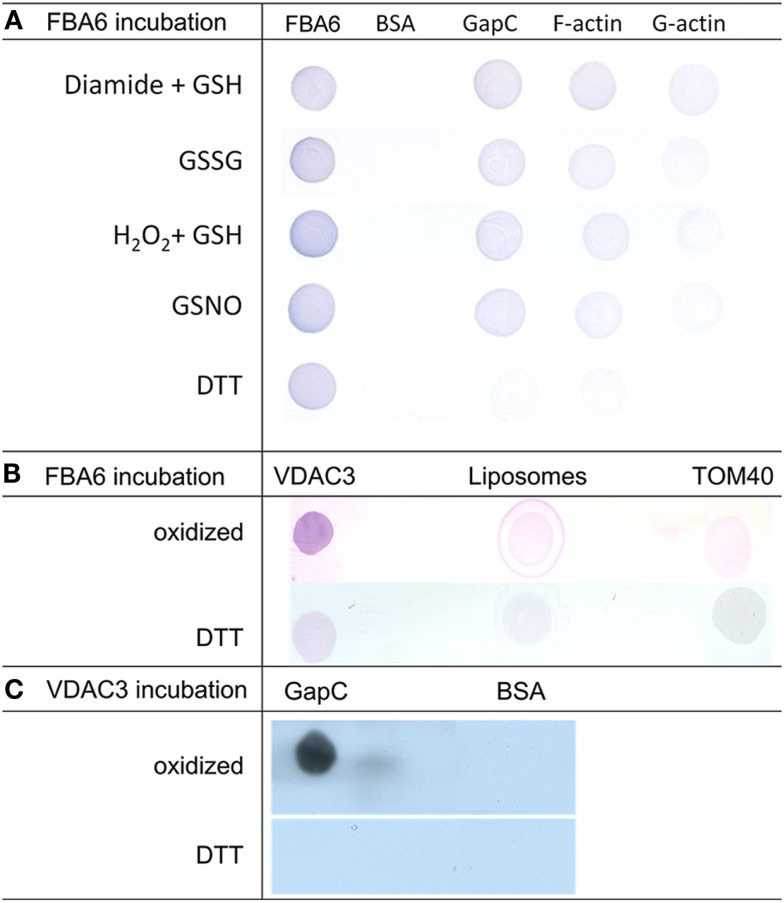
Figure 7.
Analysis redox-dependent binding of aldolase to GAPDH, actin and VDAC3 by Far Western blotting. (A) Aldolase (FBA6; At2g36460), GAPDH (GapC1; At3g04120), G-actin, rabbit F-actin, and bovine serum albumin (BSA) as negative control (2 μg each) were applied onto a nitrocellulose membrane, overlaid with 100 μg/ml aldolase in the presence of 10 mM DTT, 0.1 mM GSNO, 5 mM GSSG, 1 mM diamide plus, 1 mM GSH, or 0.5 mM H2O2 plus 0.5 mM GSH, and probed with polyclonal antibody against maize aldolase as described in Section Experimental Procedures. (B) VDAC3 (At5g15090) reconstituted into liposomes was applied onto a nitrocellulose membrane, overlaid with 100 μg/ml aldolase in the presence of 10 mM DTT and without reductant (oxidized). Empty liposomes and TOM40 were used as controls, immunological detection of bound aldolase was as in (A). (C). GapC and BSA as a control were applied to the membrane, which was overlaid with VDAC3 in liposomes. Detection was with anti-VDAC1 serum and the HRP-conjugated second antiserum using ECL as a substrate as described in Section Experimental Procedures.