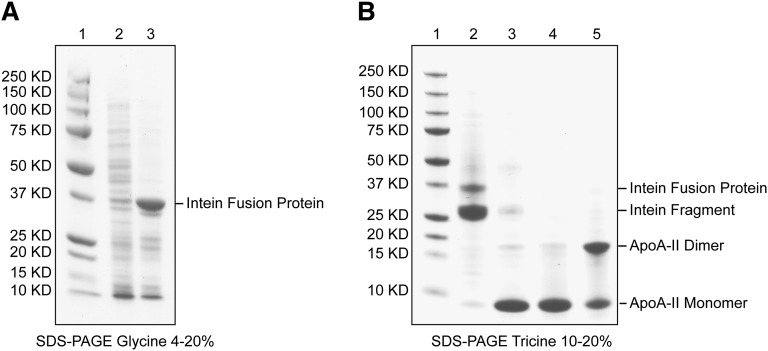
Fig. 2.
Purification summary. A: SDS-glycine PAGE gel (4–20%) stained with Coomassie blue. Lane 1: Precision Plus protein standards from Bio-Rad with indicated molecular mass; lane 2: uninduced bacterial lysate; lane 3: IPTG-induced bacterial lysate. Amplified band of intein fusion protein is marked. B: SDS-tricine PAGE gel (10–20%) stained with Coomassie blue. Lane 1: Precision Plus protein standards from Bio-Rad; lane 2: chitin beads after activation of intein auto-cleavage reaction and column elution; lane 3: eluate from the chitin column after activation of intein auto-cleavage reaction; lanes 4 and 5: flow through fraction from a Ni-chelating affinity column in which residual fusion protein has been removed; reducing and nonreducing conditions, respectively. The final purity of recombinant apoA-II is >95% by combined SDS-PAGE and mass spectrometric analyses.