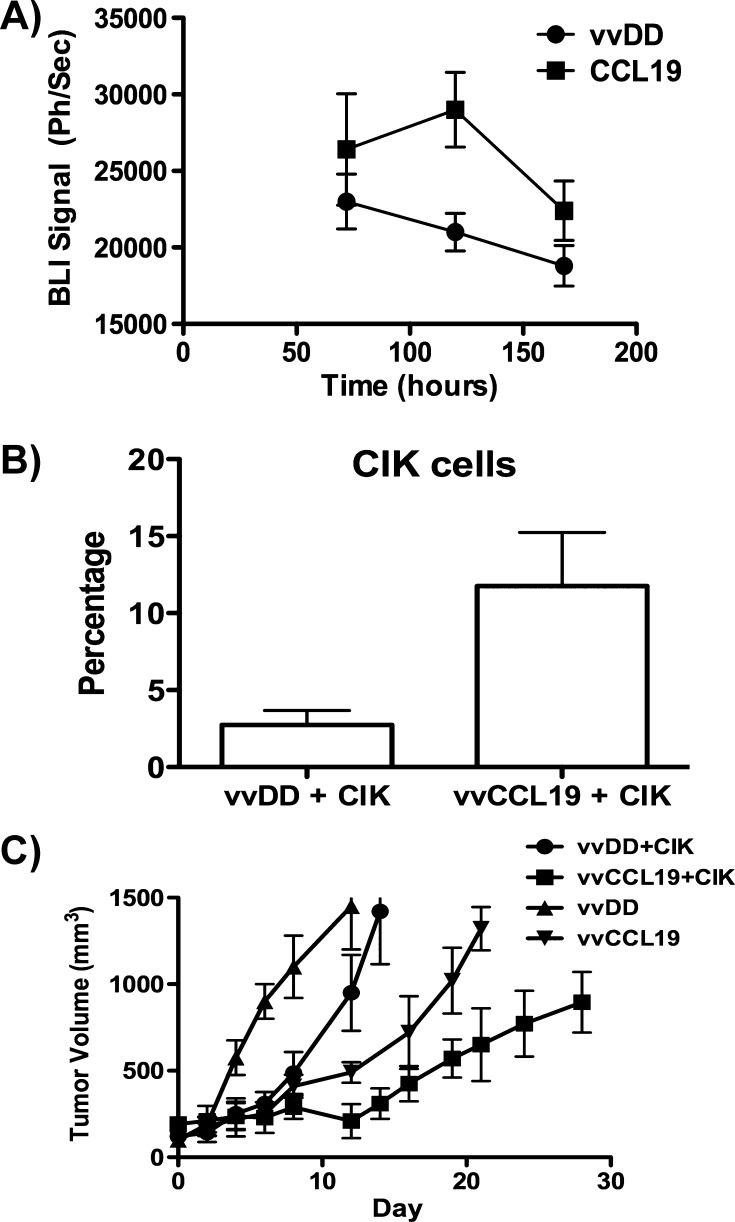
Figure 5.
(A) Adoptively transferred immune cells preferentially traffic to tumors treated with vaccinia expressing CCL19. Mice (FVB bearing bilateral subcutaneous 6780 tumors on either flank, n = 5) received virus (1 x 108 PFU) via intratumoral injection, with vvDD on one flank and vvCCL19 on the opposite flank 24 hours after injection with CIK cells expressing luciferase (expanded from a transgenic luciferase expressing mouse) via tail vein injection. Subsequent trafficking of CIK cells to each flank tumor was quantified at times after injection by bioluminescence imaging (P < .05 at 72 and 120 hours). (B) vvCCL19 preferentially attracts CIK cells into the tumor. Mice (C57/BL6) with subcutaneous MC38 tumors received IP injection of vvDD or vvCCL19 (1 x 108 PFU, n = 8 per group), followed by 1 x 108 CIK cells prelabeled with cell tracker 72 hours later. After a further 72 hours, mice were sacrifice and tumors were recovered and dissociated, and the percentage of transferred CIK cells in the tumor was determined by flow cytometry (P > .01). (C) vvCCL19 in combination with CIK cells displayed enhanced therapeutic benefit. Mice (C57/BL6) with subcutaneous MC38 tumors received IP injection of vvDD or vvCCL19 (1 x 108 PFU, n = 8 per group), followed by 1 x 108 CIK cells or PBS 72 hours later. Antitumor effect was followed by caliper measurement (P < .05 at all times after day 10 for vvCCL19 and CIK group compared to all other groups.)