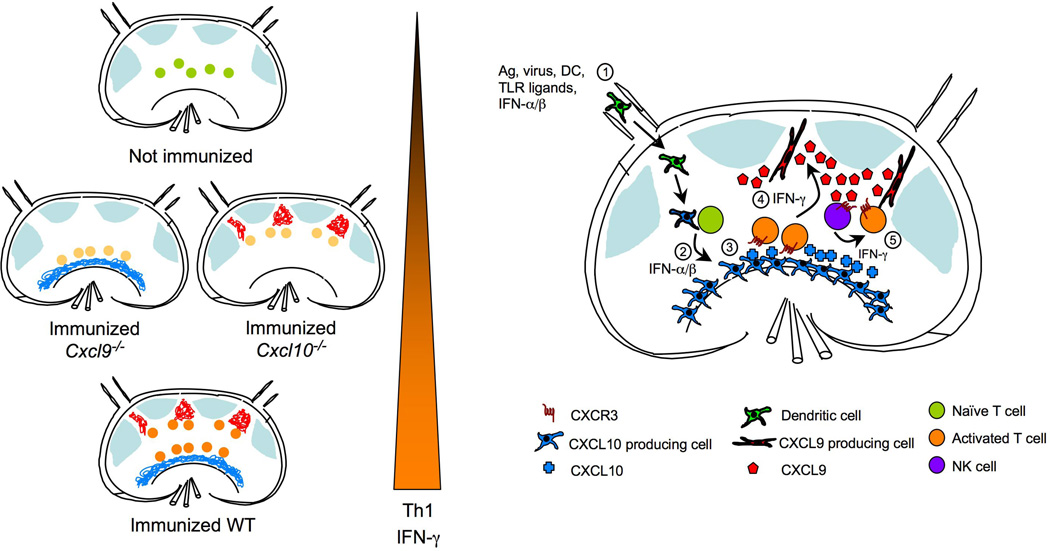
Figure 1.
Expression of CXCR3 ligands within distinct LN compartments drives optimal Th1 cell-associated IFN-γ production. Left panel is a simplified depiction of re-localization of antigen-specific T cells after activation in relation to LN compartments that express CXCL9 or CXCL10. T cells become fully activated and express highest amounts of IFN-γ only if both CXCL9 and CXCL10 are present. Right panel shows temporal and spatial expression of CXCR3 ligands in response to immunization. 1) Afferent lymphatics bring in activated DC bearing antigen, or soluble antigen, TLR ligands, and type I IFN from infected tissue; 2) While innate stimuli induce expression of CXCL10 in mostly hematopoietic cells in the medullary region, DC activate antigen-specific T cells in the T-zone; 3) Activated T cells upregulate CXCR3 expression, which promotes frequent and prolonged encounters with CXCL10 expressing antigen presenting cells (APC’s) either in the T zone or the medullary region; 4) IFN-γ produced by activated T cells induces CXCL9 expression by unidentified stromal cells in the interfollicular region; 5) CXCL9 may attract other CXCR3 expressing cells, such as NK cells, as well as activated antigen-specific CD4 T cells to the interfollicular areas leading to more exposure to IFN-γ and other cytokines resulting in optimal Th1 cell differentiation.