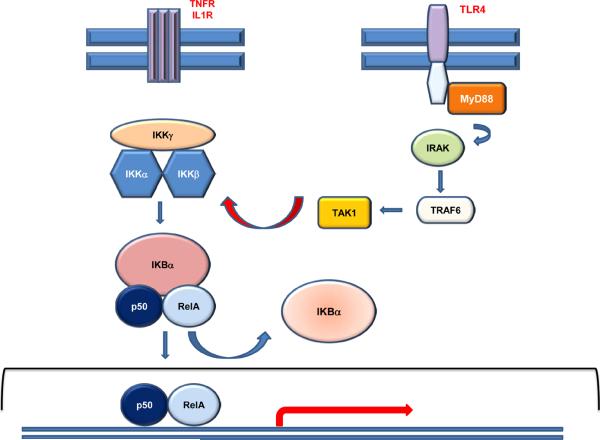
Figure 1. NF-κB signaling.
Binding of TNFα (or IL-1β) to their respective receptors triggers canonical NF-κB signaling. Signaling is initiated by activation of an IκB kinase (IKK) complex consisting of catalytic kinase subunits (IKKα and/or IKKβ) and the regulatory scaffold protein IKKγ. IKK-mediated phosphorylation results in proteasomal degradation of the IκB inhibitor enabling the active NFκB transcription factor subunits to translocate to the nucleus and induce target gene expression. One of the target genes is IκBα gene itself, which re-sequesters NFκB subunits and terminates transcriptional activity, unless a persistent activation signal is present. NF-κB is activated also through TLR signaling via Myd88 dependent pathway. Receptor induced association of MyD88 with the TIR domain allows the formation of a complex, including IRAK1 and TRAF6, resulting in activation of TAK1, which phosphorylates and activates IKKβ. TLR3 can also induce NFκB in a MyD88 independent manner (not shown).