Abstract
In classic Stroop paradigms, increasing the proportion of control-demanding incongruent trials results in strategic adjustments in behavior and implementation of cognitive control processes. We manipulated expectancy for incongruent trials in an emotional facial Stroop task to investigate the behavioral and neural effects of proportion manipulation in a cognitively demanding task with emotional stimuli. Subjects performed a high expectancy (HE) task (65% incongruent trials) and a low expectancy (LE) task (35% incongruent trials) during functional magnetic resonance imaging (fMRI). As in standard Stroop tasks, behavioral interference was reduced in the emotional facial Stroop HE task compared to the LE task. Functional MRI data revealed a switch in cognitive control strategy, from a reactive, event-related activation of a medial and lateral cognitive control network and right amygdala in the LE task to a proactive, sustained activation of right dorsolateral prefrontal cortex (DLPFC) in the HE task. Higher trait anxiety was associated with impairment (slower response time and decreased accuracy) as well as reduced activity in left ventrolateral prefrontal cortex, anterior insula, and orbitofrontal cortex in the HE task on high conflict trials with task-irrelevant emotional information, suggesting that individual differences in anxiety may be associated with expectancy-related strategic control adjustments, particularly when emotional stimuli must be ignored.
Keywords: amygdala, anterior cingulate cortex, conflict monitoring, fMRI, prefrontal cortex, sustained and transient control
1. Introduction
Cognitive control, or the ability to flexibly adapt behavior and direct cognitive processing to achieve a particular goal, is a topic of high interest in cognitive neuroscience that has been extensively studied using experimental cognitive paradigms and functional neuroimaging. For example, the classic color-word Stroop task has been utilized to reveal the behavioral and neural correlates of cognitive control. In this task, the ink color in which a color word is printed must be named. For congruent (C) trials, the word-reading and ink-color naming produce the same response (“red” written in red ink), and responses are typically fast and accurate. For incongruent (I) trials, the word-reading and ink color-naming produce different responses (“red” written in blue ink). Responses are slower and less accurate, as it is difficult to suppress the automatic word-reading response (Dalrymple-Alford & Budayer, 1966; C. M. MacLeod, 1991; Sichel & Chandler, 1969; Stroop, 1935). Early positron emission tomography (PET) imaging studies showed strong activity in dorsal anterior cingulate cortex (dACC) for the I-C contrast (Carter, Mintun, & Cohen, 1995; Pardo, Pardo, Janer, & Raichle, 1990).
In addition to this basic congruency interference effect, both congruency proportion manipulation and previous trial congruency have characteristic effects on behavior and neural activity. When a high proportion of trials within a task block are I trials, the congruency interference effect is much reduced, in both reaction time (RT) and accuracy, compared to task blocks where I trials are infrequent (Lindsay & Jacoby, 1994; Logan & Zbrodoff, 1979; Tzelgov, Henik, & Berger, 1992). In a Stroop task with only 20% I trials, subjects showed more activity in dACC in response to I trials in comparison to a Stroop task with 80% I trials (Carter et al., 2000).
Incongruent trials preceded by incongruent trials (iI trials) are often faster and more accurate than incongruent trials preceded by congruent trials (cI trials) (Egner & Hirsch, 2005; Gratton, Coles, & Donchin, 1992; Kerns et al., 2004). Event-related neuroimaging studies have shown that cI trials are characterized by increased activity in dACC, while iI trials show reduced dACC activity and increased activity in dorsolateral prefrontal cortex (DLPFC) and posterior processing regions (M. Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Egner & Hirsch, 2005; Kerns et al., 2004).
One comprehensive theory that accounts for these behavioral and neuroimaging results is conflict theory (M. M. Botvinick, Braver, Barch, Carter, & Cohen, 2001). Performance is worsened on I trials due to the preparation of two conflicting responses. In these cases, a conflict monitor is activated, which recruits cognitive control. Cognitive control enhances relevant task-processing and attention, reducing conflict and improving performance. Because the dACC is most active on cI trials (M. Botvinick et al., 1999; Kerns et al., 2004), when conflict is high, performance is poor, and control has not yet been implemented, the dACC is most likely a locus of conflict monitoring. The DLPFC, on the other hand, is more active on iI trials, and is most likely involved in the implementation of cognitive control (Egner & Hirsch, 2005; Kerns et al., 2004). Conflict theory can also account for the consequences of proportion manipulation. When there are few I trials, cognitive control is not engaged. Thus, when an I trial does occur, performance is disrupted due to the presence of conflict, and the dACC is activated. When I trials are more frequent, cognitive control is sufficiently engaged as many I trials occur. The dACC is less active and performance on I trials is less disrupted (Carter et al., 2000).
Not surprisingly, Stroop tasks and other response-conflict tasks have been modified to investigate the behavioral and neural effects of emotional stimuli on conflict monitoring and implementation of cognitive control. Early emotional Stroop tasks utilized valenced words (Whalen et al., 1998), and more recently, emotional faces (Egner, Etkin, Gale, & Hirsch, 2008; Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; Krug & Carter, 2010). Two studies using a facial Stroop task have nicely investigated trial-to-trial adjustments in an emotional task (Egner et al., 2008; Etkin et al., 2006). In both studies, iI trials were faster than cI trials. However, in Etkin et al. (2006), cI trials activated amygdala and dorsomedial prefrontal cortex (PFC), while iI trials activated rostral anterior cingulate cortex (rACC), suggesting that a different network is involved in conflict detection and control in emotional facial Stroop tasks. In Egner et al. (2008), cI trials in both an emotional task and a non-emotional task activated dACC, while iI trials in the emotional task once again activated rACC, suggesting that dACC is a common conflict detector for both emotional and non-emotional tasks, with the recruitment of a different prefrontal control region (rACC) in the emotional task. However, Krug & Carter (2010) found an overlapping dACC/DLPFC network for I trials when comparing an emotional and a non emotional-facial Stroop task. In this experiment an investigation of individual differences in trait anxiety revealed reduced behavioral interference effects and rACC activity in an emotional facial Stroop task in subjects low in trait anxiety, suggesting that performance and neural activity in emotional cognitive control paradigms may be highly sensitive to certain individual differences. Currently, no studies using an emotional Stroop paradigm have investigated the behavioral and neural consequences of I trial proportion manipulation.
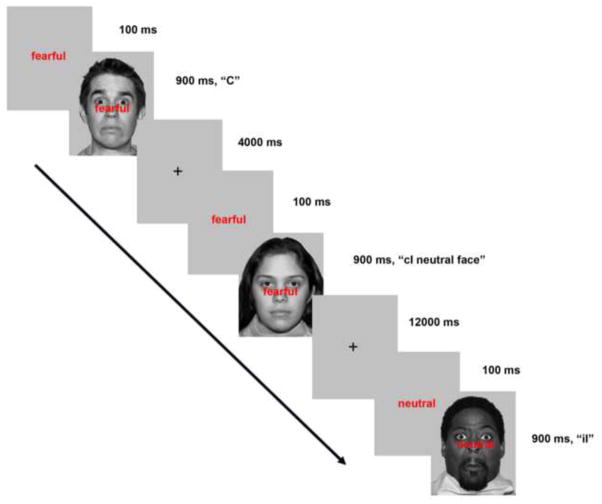
In this experiment, subjects performed two versions of an emotional facial Stroop task during fMRI scanning. In one version, the high expectancy (HE) task, 65% of trials were incongruent. All subjects also performed the low expectancy (LE) task, which had only 35% incongruent trials (Figure 1).
Figure 1.
Facial Stroop task. Subjects responded via button-press whether a face was neutral or or fearful in emotion. Each subject performed four blocks of the high expectancy task (65 % incongruent trials) and four blocks of the low expectancy task (35% incongruent trials). ms = millisecond; C = congruent trial, cI = incongruent trial preceded by congruent trial; iI = incongruent trial preceded by incongruent trial.
Goal 1: Determine the behavioral and neural consequences of incongruent trial proportion in an emotional facial Stroop task
Our first goal was to compare basic interference effects (I vs. C) between the two emotional facial Stroop tasks. Behaviorally, there should be a reduction of interference as expectancy for I trials increases. We have found that our LE emotional facial Stroop task activates a nearly identical conflict and control network compared to a LE non-emotional Facial Stroop task (Krug & Carter, 2010). Consequently, we predict similar results to those reported by Carter et al. (2000), in that the LE emotional facial Stroop task will engage dACC more strongly on I trials than the HE emotional facial Stroop task. However, we cannot be certain of this prediction as there is still substantial evidence that emotional facial Stroop tasks may recruit other regions to detect and resolve conflict (Egner et al., 2008; Etkin et al., 2006). There is also the possibility that a different mechanism may govern behavioral proportion manipulation effects in emotional facial Stroop tasks.
Goal 2: Investigate whether the high expectancy and low expectancy tasks differ in sustained control
Our second goal was to test the hypothesis that sustained control related activity would be observed in prefrontal cortex, and associated with a high expectancy of I trials. Conflict theory accounts for the behavioral and neural characteristics of proportion manipulation effects solely through trial-to-trial adjustments in conflict detection and implementation of control (M. M. Botvinick et al., 2001), the same mechanism underlying the previous trial type congruency effects shown in Gratton et al. (1992) and Kerns et al. (2004). Hence, an increase in sustained activity may not be necessary to account for the reduction in interference.
However, there is evidence that while the previous trial congruency effects are implemented via a transient control system, the behavioral effects of proportion manipulation are implemented via a separate, sustained control system (Funes, Lupianez, & Humphreys, 2010). Similarly, the dual mechanisms of control (DMC) theory suggests that cognitive control operates in two different modes. Proactive control is sustained and preparatory, while reactive control is transient and is recruited after an event occurs which requires immediate engagement of control (Braver, Gray, & Burgess, 2007). While this theory was originally developed to account for strategic differences in working memory, it can also explain the behavioral and neural effects of proportion manipulation in the color-word Stroop. De Pisapia & Braver (2006) revised Botvinick et al’s (2001) model by including two ACC/PFC conflict monitoring and control loops. One loop, specializing in reactive control, operated on a brief timescale on the order of milliseconds, while the second loop, specializing in proactive control, operated on a timescale of several seconds/minutes. Their model accounted for the behavioral effects of proportion manipulation, and also transient activation of dACC and left lateral PFC in the LE task and sustained activation of right lateral PFC in their HE task.
Other studies modeling transient and sustained activity, as well as resting state functional connectivity, suggest a transient control network that includes DLPFC and the inferior parietal lobule and a sustained control network that consists of anterior PFC, anterior insula/frontal operculum, and dACC/medial frontal cortex (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Dosenbach et al., 2007; Dosenbach et al., 2006).
We predict that if parallel processes are engaged to support cognitive control and a sustained or proactive cognitive system can support performance on tasks such as the Stroop, it will occur more strongly in the HE task. In addition to a whole brain analysis, we also performed separate small-volume ROI analyses on rACC and DLPFC (see methods), regions that are often associated with increased (event-related) control in emotional (Egner et al., 2008; Etkin et al., 2006; Krug & Carter, 2010) and non-emotional (De Pisapia & Braver, 2006; Egner et al., 2008; Egner & Hirsch, 2005; Kerns et al., 2004; Krug & Carter, 2010) tasks, and could possibly play a role, either separately or together, in sustained control in the HE condition.
Goal 3: Determine whether individual differences in trait anxiety are associated with behavior and neural activity, particularly on high conflict cI neutral face-fearful word trials
Our final objective was to look at individual differences in trait anxiety in the current experiment. Individual differences, particularly in anxiety, often contribute to performance and brain activity during emotional tasks (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van, 2007; Bishop, Duncan, Brett, & Lawrence, 2004; Dresler, Meriau, Heekeren, & van der Meer, 2009; Etkin et al., 2004; Etkin, Prater, Hoeft, Menon, & Schatzberg, 2010; Krug & Carter, 2010; Ladouceur et al., 2009; C. MacLeod & Rutherford, 1992; Mathews, Yiend, & Lawrence, 2004; Maxwell, Shackman, & Davidson, 2005; Verkuil, Brosschot, Putman, & Thayer, 2009). We decided to focus on the cI neutral-face-fearful-word trial because it may be particularly difficult for individuals with higher trait anxiety. In Krug & Carter (2010), trait anxiety was correlated with slower RT on cI neutral face-fearful word trials compared to cI fearful face-neutral word trials, perhaps because high anxiety is associated with an impaired ability to disengage from emotional stimuli (Koster, Crombez, Verschuere, & De Houwer, 2004; Salemink, van den Hout, & Kindt, 2007). In this task, the task-irrelevant word appears slightly before the task-relevant face (see methods) and is automatically processed. High anxiety subjects may have difficulty disengaging from the task-irrelevant “fearful” word, particularly when it is not expected and control processes are not yet recruited. In accordance with this view, the subjects with higher anxiety showed more dACC activity, suggesting heightened response conflict due to concurrent preparation of both the task-relevant face and the more automatic, task irrelevant word response, as well as a lack of rACC control-related activity on the cI neutral face-fearful word trial.
In this previous experiment, 30% of trials were incongruent, thus I trials were unexpected (comparable to the LE task in the present experiment). Our goal was to compare the LE and HE tasks to see if behavioral and neural differences on the cI neutral face-fearful word trial that can be explained by individual differences in trait anxiety persist when incongruent trials are expected. Even if the behavioral differences associated with trait anxiety in the LE task are eliminated in a HE context, the neural resolution of this interference may differ. Trait anxiety may also contribute to individual differences in overall interference effects and sustained activity, as there is some evidence that people high in anxiety or high in BIS (behavioral inhibition system) may be more likely to adopt a reactive/transient control strategy (Braver et al., 2007; Fales et al., 2008; Gray & Braver, 2002; Gray et al., 2005).
2. Results
Behavioral Results
Interference Effects
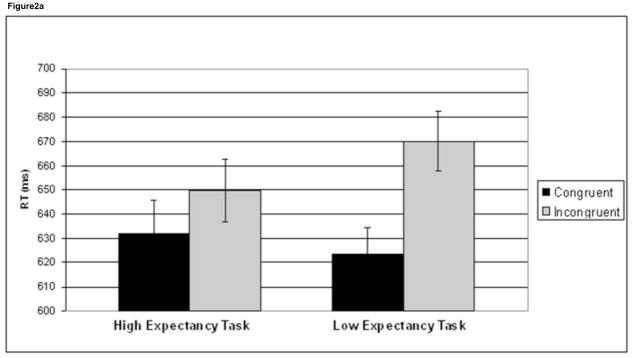
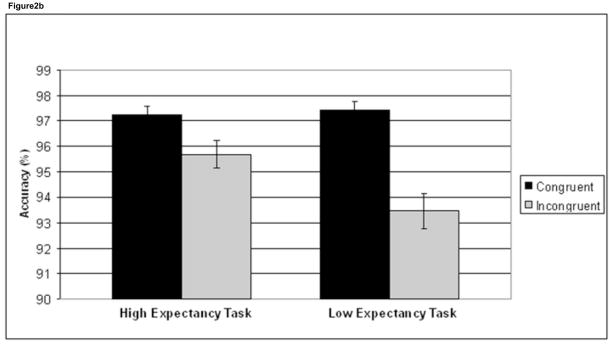
To investigate interference effects, a task (HE, LE) × trial congruency (C, I) ANOVA was performed on RT and accuracy data. This interaction was significant for both RT (F1,41 =50.9, p<.001) and accuracy (F1,41 =23.3, p<.001). Incongruent trials were significantly slower than C trials in both the HE task (t41 = 5.8, p <.001) and the LE task (t41 = 11.1, p <.001), although this interference effect was larger in the LE task (46.5 ms) compared to the HE task (17.5 ms), driving the significant task × congruency interaction (Figure 2a, Table 1). Likewise, for the accuracy data, I trials were significantly less accurate than C trials in both tasks (HE task: t41 = 4.7, p <.001; LE task: t41 = 7.1, p <.001), with a larger accuracy difference between C and I trials in the LE task (4.0%) compared to the HE task (1.5%) (Figure 2b, Table 1).
Figure 2.
Behavioral interference effects. Mean reaction time (RT) (a) and accuracy (%) (b) data are plotted for congruent trials (black) and incongruent trials (grey) in the high expectancy task and the low expectancy task. Incongruent trials are significantly slower and less accurate than congruent trials in both tasks. There was also a task × congruency interaction in both RT and accuracy, driven by greater interference effects in the low expectancy task. Error bars indicate standard error of the mean (SEM).
Table 1.
Mean Behavioral Measures in Reaction Time and Accuracy for High Expectancy and Low Expectancy Task
| High Expectancy Task | Low Expectancy Task | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| RT: | %: | RT: | %: | |||||
| Trial Type | M | (SD) | M | (SD) | M | (SD) | M | (SD) |
| Incongruent | 649.8 | (83.9) | 95.7 | (3.5) | 670.0 | (81.0) | 93.5 | (4.5) |
| Congruent | 632.3 | (87.1) | 97.2 | (2.3) | 623.5 | (70.7) | 97.4 | (2.2) |
| cI | 650.3 | (86.4) | 95.0 | (4.3) | 667.2 | (76.5) | 92.9 | (5.0) |
| iI | 649.5 | (83.0) | 96.0 | (3.5) | 673.6 | (88.7) | 94.3 | (4.6) |
| cC | 627.4 | (81.5) | 97.0 | (2.5) | 618.5 | (69.8) | 97.5 | (2.1) |
| iC | 636.1 | (93.5) | 97.4 | (3.0) | 635.3 | (74.4) | 97.2 | (2.8) |
| cI neutral face | 659.1 | (91.4) | 95.8 | (3.6) | 684.7 | (79.8) | 94.0 | (5.9) |
| cI fearful face | 640.8 | (88.2) | 94.2 | (6.6) | 649.8 | (76.7) | 91.8 | (6.4) |
Note. Mean RT data are for correct trials only, in milliseconds.
RT = reaction time; % = percent correct; cI = incongruent trial preceded by congruent trial; iI = incongruent trial preceded by incongruent trial; cC = congruent trial preceded by congruent trial; iC = congruent trial preceded by incongruent trial.
In addition to looking at the difference between C and I trials, we also investigated C and I trials separately. Incongruent trials were significantly faster in the HE task (649.8 ms) compared to the LE task (670.0 ms), t41 = 3.6, p =.001. Incongruent trials were significantly more accurate in the HE condition (95.7%) compared to the LE task (93.5%), t41 = 5.5, p <.001 (Table 1). Congruent trials for the two tasks did not differ in RT or accuracy. Thus, behavioral interference effects were mainly driven by changes in performance on I trials.
Trial to Trial Effects
To investigate conflict adaptation effects we tested for a previous trial congruency (c, i) by current trial congruency (C, I) interaction. We also directly compared performance on cI and iI trials, and cC and iC trials.
For RT in the HE task, there was a trend toward significance for the previous trial by current trial interaction (F1,41 =3.6, p=.065), driven by a trend for slower RT on iC trials compared to cC trials (t41 = 1.9, p =.063). For the LE task, the previous trial by current trial congruency ANOVA was significant in RT (F1,41 =7.1, p=.011), driven by iC trials that were significantly slower than cC trials (t41 = 5.5, p <.001) (Table 1).
For accuracy, in the HE task the previous trial × current trial interaction was not significant, although there was some evidence of conflict adaptation as there was a strong trend for higher accuracy on iI trials compared to cI trials (t41 = 2.0, p =.054). In the LE task, the previous × current trials interaction was significant (F1,41 =6.1, p=.018), and driven by higher accuracy on iI trials compared to cI trials (t41 = 2.3, p =.026) (Table 1).
When these conflict adaptation effects were directly compared between tasks, no significant differences were found in RT or accuracy.
Emotion and Conflict
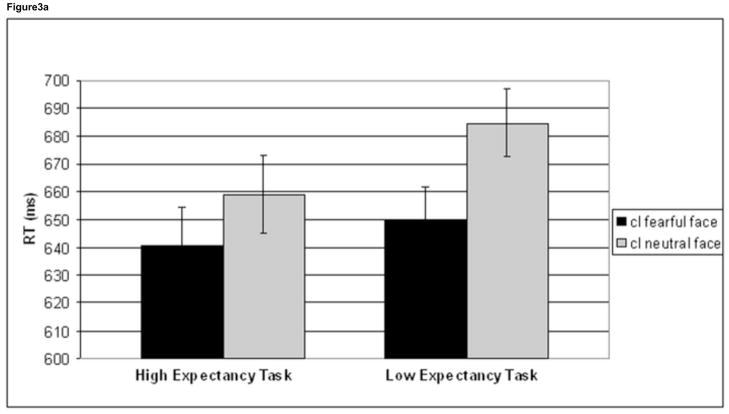
We investigated the effects of emotion on high conflict (cI) trials. For cI trials a task (HE task, LE task) by face (cI fearful face, cI neutral face) interaction approached significance for RT (F1,41 =3.9, p=.056), and was driven by a greater difference between these two trial types in the LE task (34.9 ms) compared to the HE task (18.3 ms) (Figure 3a). Follow-up tests showed that cI neutral face trials were significantly slower than cI fearful face trials in both tasks (HE task: t41 = 2.5, p =.018; LE task: t41 = 6.8, p <.001). Additionally, cI neutral face trials in the HE task were significantly faster than cI neutral face trials in the LE task (t41 = 3.3, p =.002, 25.5 ms difference), while there was no difference in RT for cI fearful face trials between the two tasks (t41 = 1.3, p =.205). A task x face ANOVA was not significant in the accuracy domain (F1,41 =.172, p=.680) (Table 1).
Figure 3.
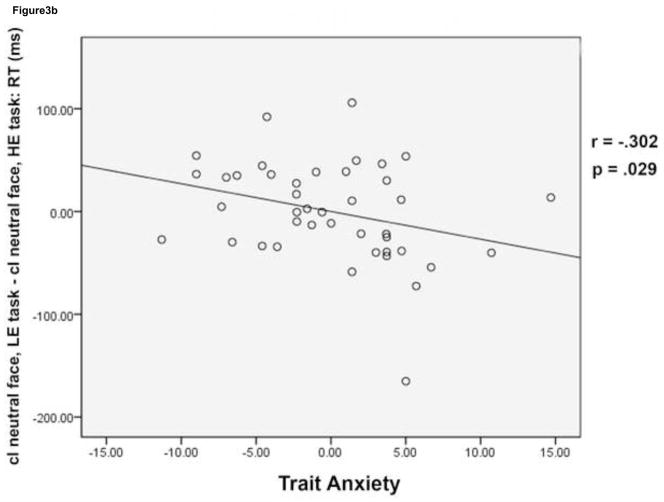
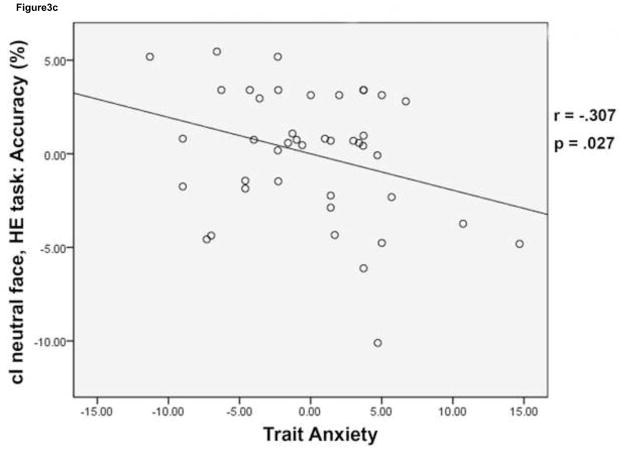
Behavioral effects of conflict and emotion. Mean reaction time (RT) is plotted for cI fearful face trials (black) and cI neutral face trials (grey) in the HE task and the LE task (a). While cI neutral face trials were slower than cI fearful face trials in both tasks, a task (HE task, LE task) by face (cI fearful face, cI neutral face) interaction was driven by an improvement in RT for cI neutral face trials in the HE task compared to the LE task. Error bars indicate standard error of the mean (SEM). For each subject, mean RT for cI neutral face trials, LE task – mean RT for cI neutral face trials, HE task was plotted against trait anxiety score (b). High anxiety was negatively correlated with this behavioral measure, showing that lower anxiety is associated with greater RT improvement on cI neutral face trials in the HE task. Mean % accuracy for the cI neutral face trial in the HE task was plotted against trait anxiety score (c). High anxiety was negatively correlated with this behavioral measure as well, showing that lower anxiety is associated with higher accuracy on this trial type in the HE task. Correlations were partial correlations, controlling for task order and task version (see methods). HE = high expectancy task; LE = low expectancy task; cI = incongruent trial preceded by congruent trial.
Individuals Differences in Trait Anxiety
We performed correlations to see if interference effects in RT and accuracy are correlated with trait anxiety. Partial correlations between trait anxiety and behavioral measures were performed, controlling for task order and task version. While the high number of correlations performed increases risk of Type I error, all comparisons were planned as we predicted that increased interference in RT and accuracy in each of the two tasks, and/or decreased reduction in interference in the HE task compared to the LE task, could be correlated with higher levels of trait anxiety.
The difference in RT between I and C trials (IRT-CRT) was not significantly correlated with trait anxiety in either the HE task or the LE task. Likewise, in the accuracy domain, the difference in % accuracy for the C trials compared to I trials (C % accuracy – I % accuracy) was not significantly correlated with trait anxiety score in either of the two tasks.
We looked at behavioral measures for I trials alone. Reaction time for I trials did not correlate with anxiety for either of the tasks. Likewise, accuracy measures for I trials did not correlate with anxiety in either the HE task or the LE task. However, the difference in RT for I trials (I, LE task-I, HE task) was negatively correlated with trait anxiety (r = −.307, p = .027). The difference in accuracy for I trials (I, HE task – I, LE task) was also negatively correlated with trait anxiety (r = −.273, p = .045).
Most importantly, we focused our analyses of individual differences on cI neutral face trials, which caused behavioral interference in the group as a whole, particularly in the LE task (see above). We correlated RT and accuracy data with trait anxiety score to investigate whether disruption in performance as a result of conflict and emotion is greater in individuals who score higher in trait anxiety. Reaction time for cI neutral face trials did not correlate with trait anxiety for either of the tasks. However, RT adjustment (cI neutral face, LE task – cI neutral face, HE task) was negatively correlated with trait anxiety (r = −.302, p = .0291) (Figure 3b). Accuracy on cI neutral face trials during the HE task was negatively correlated with trait anxiety (r = −.307, p = .0272) (Figure 3c).
Three of our four significant correlations involve a behavioral difference score ((I, LE task-I, HE task, RT); ((I, HE task-I, LE task, accuracy); (cI neutral face, LE task – cI neutral face, HE task). Thus, there are two possible interpretations: 1) low anxiety is correlated with better performance on the HE task compared to the LE task, or 2) low anxiety is correlated with worse performance on the LE task compared to the HE task. We did a median split, based on trait anxiety score, to create a high anxiety (HA) and low anxiety (LA) group to distinguish between these two possibilities. HA and LA subjects had nearly identical I RT (HA = 669.6 ms; LA = 670.3 ms) and cI neutral face RT in the LE task (HA = 684 ms; LA = 685 ms). However, the LA group was significantly faster on I trials in the HE task compared to the LE task (t20 = 4.444, p < .001; 28 ms faster) and also on cI neutral face trials in the HE task compared to the LE task (t20 = 5.821, p <.001; 38 ms faster) while the HA subjects were not significantly faster on I trials (t20 = 1.350, p = .192; only 13 ms faster) or cI neutral face trials (t20 = .965, p = .346; only 13 ms faster) for the HE task compared to the LE task. Likewise, LA and HA subjects had nearly identical I accuracy for the LE task (HA = 93.4%; LA = 93.6%). The LA group was significantly more accurate for I trials in the HE task compared to the LE task (t20 = 6.277, p < .001; 3.0% more accurate) while the HA subjects were also more accurate on I trials, although this difference in accuracy was smaller (HE task compared to the LE task (t20 = 2.318, p = .031; only 1.4% more accurate). This suggests that lower anxiety is correlated with improvement on the HE task compared to the LE task, and not that lower anxiety is correlated with impaired performance on the LE task.
fMRI Results
Analysis 1: Interference Effects
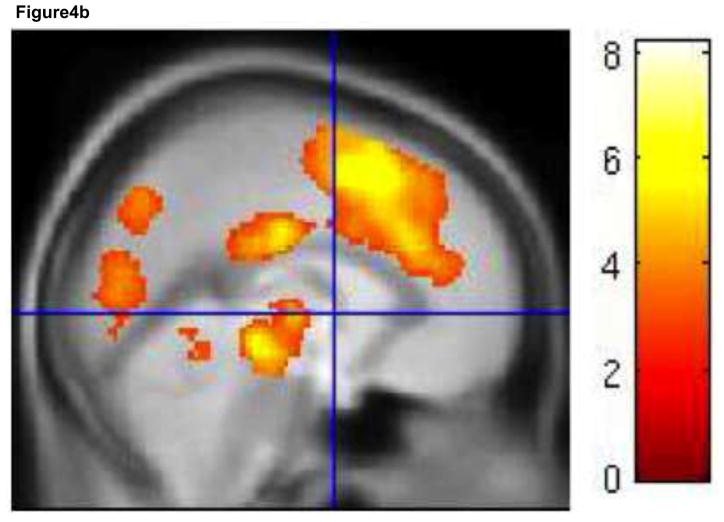
For the HE task, an I-C contrast did not show any activity that survived correction for multiple comparisons. Even at a liberal threshold of p<.005, uncorrected, there was no evidence of activity in medial or lateral conflict/control regions. For the LE task, a cluster extent threshold of 377 voxels at p<.005 was necessary to correct for multiple comparisons. A large cluster of over 50,000 voxels was significant, and included dACC (extending into supplementary motor cortex), insula, dorsal and ventral lateral PFC (BA 9, 46, 45, 47) and inferior parietal cortex, an extensive network of regions involved in conflict detection and implementation of cognitive control (Figure 4a, b, Table 2).
Figure 4.
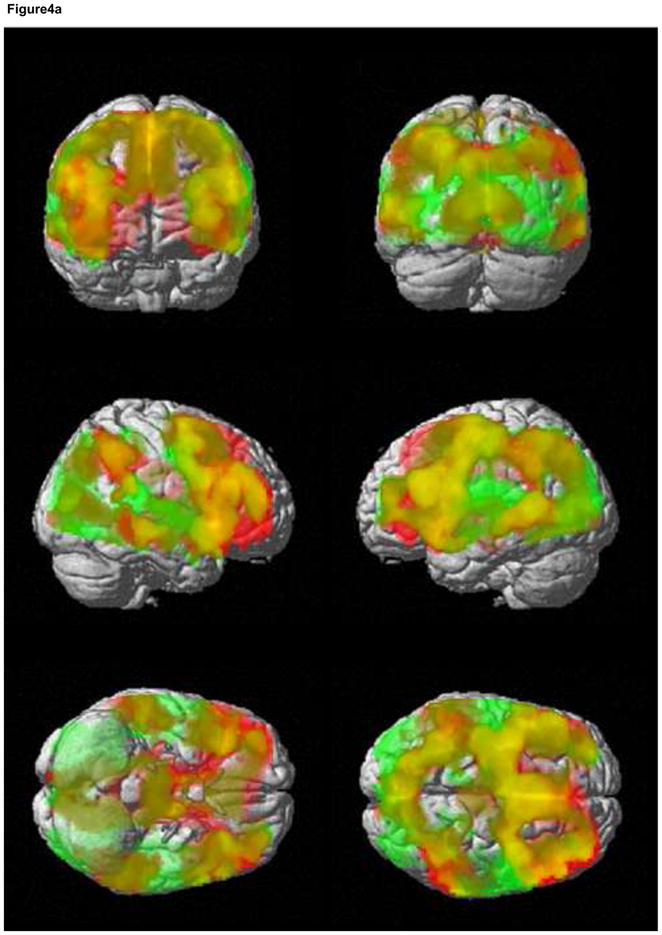
Interference activation. Incongruent trials activate an extensive medial and lateral cognitive control network in the LE task. Activity for the contrast I-C, LE task is shown in red. Activity for the contrast (I-C, LE task) – (I-C, HE task) is shown in green. Areas of overlap are shown in yellow (a). There were no areas of activation for the contrast I-C, HE task. Medial views of I-C, LE task (b) and (I-C, LE task) – (I-C, HE task) (c) are shown separately. Results are displayed at p < .005, with a cluster extent threshold that corrects for multiple comparisons (p<.05) (see methods). For (b) and (c), colorbar indicates T statistic. HE = high expectancy task; LE = low expectancy task; I = incongruent trial; C = congruent trial.
Table 2.
fMRI Summary Data- Analysis 1: Interference and Trial-to-Trial Effects
| a. Whole Brain
| ||||||
|---|---|---|---|---|---|---|
| Contrast | Cluster Extent Threshold | # Voxels | Peak T Value | Peak MNI Coordinates x, y, z | Anatomical Area(s) | Brodmann Area(s) |
| I-C, LE task | 377 | 50, 640 | 8.17 | 34 20 −12 | IFG | 47 |
| 8.00 | 38 18 6 | Insula | 13 | |||
| 7.39 | −4 −20 32 | Cingulate gyrus | 23 | |||
| 7.34 | −6 4 54 | MFG | 6 | |||
| 6.84 | 10 24 30 | Cingulate gyrus | 32 | |||
| 6.62 | −34 14 6 | Insula | 13 | |||
| 6.60 | −32 22 −4 | IFG | 47 | |||
| 6.47 | −46 16 22 | IFG | 45 | |||
| 6.46 | −32 16 −14 | IFG | 47 | |||
| 6.40 | 8 22 42 | Cingulate gyrus | 32 | |||
| 6.37 | 40 22 18 | IFG | 45 | |||
| 6.32 | 8 32 28 | Anterior cingulate | 24 | |||
| 6.31 | 2 20 52 | Medial FG | 32 | |||
| 6.28 | −40 −2 38 | Precentral gyrus | 8 | |||
| 6.16 | −28 −6 54 | MFG | 6 | |||
| 6.10 | −30 −50 36 | IPL | 40 | |||
| 5.95 | 48 26 22 | MFG | 46 | |||
| 5.94 | −4 −28 −12 | Midbrain | ||||
| 5.93 | −50 24 24 | IFG | 45 | |||
| 5.89 | −40 4 28 | IFG | 9 | |||
| (I-C, LE task)-(I-C, HE task) | 385 | 45,036 | 6.18 | −48 −48 10 | STG | 22 |
| 6.13 | 18 −62 4 | Posterior cingulate | 30 | |||
| 5.81 | 18 −68 14 | Posterior cingulate | 31 | |||
| 5.55 | −10 −8 −8 | Midbrain | ||||
| 5.41 | −16 −72 6 | Cuneus | 30 | |||
| 5.41 | 36 20 2 | Insula | 45 | |||
| 5.26 | 8 22 30 | Anterior cingulate | 24 | |||
| 5.25 | 12 6 50 | Cingulate gyrus | 24 | |||
| 5.25 | −8 −2 58 | Medial FG | 6 | |||
| 5.14 | −30 24 6 | Insula | 45 | |||
| 5.14 | −10 18 34 | Cingulate gyrus | 32 | |||
| 5.12 | −36 26 −2 | IFG | 47 | |||
| 5.08 | −22 −66 −8 | Fusifrom gyrus | 19 | |||
| 5.05 | 38 2 50 | MFG | 6 | |||
| 5.04 | −62 2 22 | Precentral gyrus | 6 | |||
| 5.03 | −44 16 22 | MFG | 46 | |||
| 5.02 | −12 −58 −8 | Cerebellum | ||||
| 4.99 | −2 −28 −8 | Midbrain | ||||
| 4.99 | −50 26 26 | MFG | 46 | |||
| 4.95 | −8 −80 12 | Cuneus | 18 | |||
| cI-iI, HE task | 348 | 523 | 5.73 | −40 −40 −10 | Parahippocampal gyrus | 19 |
| 4.18 | −46 −18 −20 | ITG | 20 | |||
| 4.12 | −34 −24 −8 | Caudate | ||||
| 3.89 | −44 −46 −4 | MTG | ||||
| 3.85 | −42 −30 −14 | Parahippocampal gyrus | 36 | |||
| 3.26 | −40 −44 −4 | Parahippocampal gyrus | 19 | |||
| 828 | 5.02 | 46 −70 30 | Angular gyrus | 39 | ||
| 4.66 | 46 −62 22 | MTG | 39 | |||
| 4.34 | 46 −48 26 | IPL | 40 | |||
| 3.07 | 42 −52 16 | STG | 22 | |||
| 2,524 | 4.83 | 2 −66 52 | Precuneus | 7 | ||
| 4.72 | 4 −62 60 | Precuneus | 7 | |||
| 4.33 | 12 −26 64 | Medial FG | 6 | |||
| 4.28 | 4 −78 52 | Precuneus | 7 | |||
| 4.08 | 8 −56 72 | Postcentral gyrus | 7 | |||
| 4.04 | −2 60 46 | SFG | 9 | |||
| 4.00 | 10 −34 64 | Paracentral lobule | 6 | |||
| 3.98 | 4 −46 64 | Paracentral lobule | 5 | |||
| 3.96 | −4 −54 70 | Postcentral gyrus | 7 | |||
| 3.89 | −4 −38 76 | Paracentral lobule | 6 | |||
| 3.82 | 10 −30 72 | Medial FG | 6 | |||
| 3.60 | 16 −20 74 | Precentral gyrus | 6 | |||
| 3.56 | 20 −24 62 | Precentral gyrus | 4 | |||
| 3.37 | −4 −30 74 | Medial FG | 6 | |||
| 3.35 | 2 −50 56 | Precuneus | 7 | |||
| 3.25 | 18 −34 66 | Medial FG | 4 | |||
| 3.19 | 4 −22 70 | Medial FG | 6 | |||
| 3.16 | 14 −44 64 | Postcentral gyrus | 3 | |||
| 3.07 | 18 −8 72 | SFG | 6 | |||
| 3.00 | −14 −44 74 | Postcentral gyrus | 3 | |||
| 849 | 4.55 | 24 22 36 | Medial FG | |||
| 4.17 | 34 10 54 | MFG | 6 | |||
| 3.98 | 28 16 50 | MFG | 8 | |||
| 3.74 | 12 38 44 | Medial FG | 8 | |||
| 3.64 | 22 12 38 | Cingulate gyrus | 32 | |||
| 3.56 | 24 12 56 | SFG | 6 | |||
| 3.49 | 16 24 20 | Caudate | ||||
| 3.41 | 16 26 44 | Medial FG | 8 | |||
| 3.34 | 30 28 28 | MFG | ||||
| 3.23 | 20 30 28 | Medial FG | 9 | |||
| 3.13 | 26 16 28 | MFG | ||||
| 3.05 | 4 46 42 | Medial FG | 6 | |||
| 2.84 | 8 36 52 | SFG | 8 | |||
| 2.82 | 32 0 64 | MFG | 6 | |||
| 468 | 4.49 | 10 48 −14 | SFG | 11 | ||
| 3.47 | 18 34 6 | Anterior cingulate | ||||
| 3.34 | 18 50 4 | Anterior cingulate | 10 | |||
| 3.22 | 18 42 0 | Anterior cingulate | 32 | |||
| 3.16 | 14 34 −16 | IFG | 47 | |||
| 3.14 | 10 32 −2 | Anterior cingulate | 32 | |||
| 1,163 | 4.13 | 14 −42 26 | Cingulate gyrus | 31 | ||
| 3.98 | 2 −50 20 | Posterior cingulate | 30 | |||
| 3.71 | 10 −36 14 | Thalamus | ||||
| 3.63 | 14 −48 6 | Posterior cingulated | 29 | |||
| 3.42 | 16 −20 20 | Thalamus | ||||
| 3.39 | 22 −26 20 | Caudate | ||||
| 3.25 | 14 −28 14 | Thalamus | ||||
| 3.07 | −2 −44 30 | Cingulate gyrus | 31 | |||
| 2.95 | −4 −66 16 | Posterior cingulate | 23 | |||
| 2.92 | 12 −48 16 | Posterior cingulate | 29 | |||
| 2.89 | −8 −50 6 | Posterior cingulate | 29 | |||
| 2.88 | 6 −46 6 | Posterior cingulate | 29 | |||
| 567 | 4.07 | −30 14 32 | MFG | 9 | ||
| 3.62 | −26 18 40 | MFG | 8 | |||
| 3.59 | −18 26 20 | Anterior cingulate | ||||
| 3.54 | −20 22 28 | Medial FG | 9 | |||
| 3.26 | −36 20 30 | MFG | 9 | |||
| 3.18 | −16 22 38 | Cingulate gyrus | 32 | |||
| 3.15 | −32 6 42 | Precentral gyrus | 9 | |||
| 3.09 | −24 4 44 | MFG | 6 | |||
| 3.08 | −32 2 50 | MFG | 6 | |||
| 3.02 | −30 4 26 | Precentral gyrus | 6 | |||
| 3.01 | −14 18 46 | Cingulate gyrus | 32 | |||
| 2.97 | −34 0 60 | MFG | 6 | |||
| 2.88 | −42 0 50 | MFG | 6 | |||
| 2.86 | −28 8 58 | MFG | 6 | |||
| cI, LE task– cI, HE task | 390 | 8,715 | 6.18 | 28 52 −10 | MFG | 11 |
| 5.56 | 60 18 30 | MFG | 9 | |||
| 5.53 | 38 50 −12 | MFG | 11 | |||
| 5.10 | 12 6 14 | Caudate | ||||
| 5.08 | 44 14 52 | MFG | 6 | |||
| 5.08 | 40 18 6 | Insula | 13 | |||
| 4.72 | 16 10 −12 | Lentiform nucleus | ||||
| 4.63 | 34 24 8 | IFG | 13 | |||
| 4.56 | 14 48 12 | Medial FG | 10 | |||
| 4.54 | 14 8 46 | Medial FG | 32 | |||
| 4.51 | 38 42 −16 | MFG | 11 | |||
| 4.51 | 8 28 34 | Medial FG | 9 | |||
| 4.48 | −8 4 42 | Cingulate gyrus | 32 | |||
| 4.44 | 14 14 8 | Caudate | ||||
| 4.26 | 36 26 42 | MFG | 9 | |||
| 4.22 | 10 18 44 | Cingulate gyrus | 32 | |||
| 4.18 | 28 50 32 | SFG | 10 | |||
| 3.90 | 36 26 −2 | IFG | 47 | |||
| 3.85 | −4 −16 4 | Thalamus | ||||
| 3.82 | 38 48 14 | MFG | 10 | |||
| 1,881 | 5.54 | 54 −46 50 | IPL | 40 | ||
| 5.16 | 48 −58 52 | IPL | 40 | |||
| 4.03 | 42 −52 44 | IPL | 40 | |||
| 4.00 | 54 −64 34 | Angular gyrus | 39 | |||
| 3.97 | 58 −56 24 | Supramarginal gyrus | 40 | |||
| 3.82 | 34 −52 42 | IPL | 40 | |||
| 3.45 | 50 −48 40 | IPL | 40 | |||
| 3.22 | 46 −38 44 | IPL | 40 | |||
| 3.19 | 60 −40 28 | IPL | 40 | |||
| 2.94 | 48 −34 36 | Postcentral gyrus | 2 | |||
| 2.92 | 58 −44 36 | Supramarginal gyrus | 40 | |||
| 2.92 | 34 −42 38 | IPL | 40 | |||
| 4,489 | 5.42 | −44 18 50 | MFG | 8 | ||
| 5.21 | −30 20 8 | Insula | 13 | |||
| 5.17 | −50 16 44 | MFG | 8 | |||
| 4.95 | −30 54 −4 | MFG | 10 | |||
| 4.86 | −34 20 44 | Precentral gyrus | 9 | |||
| 4.81 | −58 12 28 | IFG | 9 | |||
| 4.76 | −38 12 10 | Insula | 13 | |||
| 4.65 | −38 48 −10 | MFG | 11 | |||
| 4.48 | −10 10 12 | Caudate | ||||
| 4.28 | −54 16 4 | IFG | 45 | |||
| 4.21 | −38 50 14 | MFG | 10 | |||
| 4.20 | −60 12 18 | IFG | 44 | |||
| 4.10 | −32 24 0 | Insula | 13 | |||
| 4.04 | −46 42 −6 | MFG | 47 | |||
| 3.86 | −40 48 6 | IFG | 10 | |||
| 3.84 | −48 42 4 | IFG | 46 | |||
| 3.78 | −56 22 14 | IFG | 45 | |||
| 3.65 | −40 40 10 | IFG | 46 | |||
| 3.58 | −56 6 8 | Precentral gyrus | 44 | |||
| 3.54 | −28 22 −8 | IFG | 47 | |||
| 1,552 | 4.67 | −54 −50 48 | IPL | 40 | ||
| 4.60 | −56 −54 40 | IPL | 40 | |||
| 3.97 | −62 −46 16 | STG | 22 | |||
| 3.94 | −60 −48 4 | MTG | 21 | |||
| 3.86 | −48 −52 54 | IPL | 40 | |||
| 3.78 | −42 −66 50 | SPL | 7 | |||
| 3.78 | −56 −40 50 | IPL | 40 | |||
| 3.62 | −38 −48 48 | IPL | 40 | |||
| 3.39 | −36 −44 40 | Supramarginal gyrus | 40 | |||
| 3.17 | −62 −34 44 | IPL | 40 | |||
| 3.04 | −52 −46 34 | Supramarginal gyrus | 40 | |||
| 3.04 | −30 −54 40 | SPL | 7 | |||
| 435 | 4.05 | 8 −20 32 | Cingulate gyrus | 23 | ||
| 3.94 | −4 −24 32 | Cingulate gyrus | 23 | |||
| 3.84 | 8 −26 26 | Cingulate gyrus | 23 | |||
| 3.48 | −6 −32 26 | Posterior cingulate | 23 | |||
| 2.97 | 10 −22 46 | Cingulate gyrus | 24 | |||
| 2.94 | −14 −32 24 | Caudate | ||||
| 402 | 3.97 | 6 −70 40 | Precuneus | 7 | ||
| 3.81 | −2 −68 38 | Precuneus | 7 | |||
| 3.32 | −8 −72 30 | Cuneus | 7 | |||
| 2.95 | −10 −58 34 | Precuneus | 31 | |||
| iI, LE task– iI, HE task | 464 | 4,654 | 4.97 | 54 18 32 | MFG | 9 |
| 4.34 | 34 46 −14 | SFG | 11 | |||
| 4.32 | 40 46 6 | IFG | 46 | |||
| 4.28 | 40 8 30 | IFG | 9 | |||
| 4.23 | 44 30 32 | MFG | 9 | |||
| 4.12 | 52 32 22 | MFG | 46 | |||
| 4.11 | 28 52 −12 | MFG | 11 | |||
| 4.10 | 36 24 8 | IFG | 45 | |||
| 4.06 | 34 44 12 | MFG | 10 | |||
| 4.02 | 46 8 36 | MFG | 9 | |||
| 3.86 | 54 14 8 | Preentral gyrus | 44 | |||
| 3.83 | 52 12 16 | IFG | 44 | |||
| 3.76 | 40 30 42 | MFG | 9 | |||
| 3.65 | 48 6 48 | MFG | 6 | |||
| 3.58 | 32 −6 46 | MFG | 6 | |||
| 3.56 | 30 26 −2 | IFG | 47 | |||
| 3.52 | 46 18 4 | IFG | 45 | |||
| 3.38 | 48 14 50 | MFG | 8 | |||
| 3.36 | 26 48 28 | SFG | 10 | |||
| 3.28 | 14 46 12 | Anterior cingulate | 32 | |||
| 1,992 | 4.72 | 46 −50 40 | IPL | 40 | ||
| 4.16 | 62 −46 32 | Supramarginal gyrus | 40 | |||
| 3.98 | 60 −42 44 | IPL | 40 | |||
| 3.82 | 52 −50 34 | Supramarginal gyrus | 40 | |||
| 3.61 | 48 −54 50 | IPL | 40 | |||
| 3.55 | 16 −76 42 | Precuneus | 7 | |||
| 3.18 | 44 −50 58 | IPL | 40 | |||
| 3.15 | 56 −34 36 | IPL | 40 | |||
| 3.12 | 60 −54 14 | STG | 22 | |||
| 3.09 | 48 −38 38 | IPL | 40 | |||
| 3.08 | 32 −64 42 | SPL | 7 | |||
| 3.01 | 36 −42 42 | IPL | 40 | |||
| 638 | 4.25 | 10 20 44 | Cingulate gyrus | 32 | ||
| 3.52 | 8 26 56 | SFG | 8 | |||
| 3.28 | 12 8 46 | Medial FG | 32 | |||
| 629 | 3.98 | 2 −30 −10 | Midbrain | |||
| 3.85 | 8 −10 6 | Thalamus | ||||
| 3.40 | 12 −14 −12 | Midbrain | ||||
| 3.20 | 12 −24 8 | Thalamus | ||||
| 3.13 | −20 −26 0 | Thalamus | ||||
| 3.08 | −8 −12 6 | Thalamus | ||||
| 3.08 | 18 −12 −2 | Lentiform nucleus | ||||
| 3.06 | 22 −12 6 | Lentiform nucleus | ||||
| 3.02 | 8 −24 −18 | Midbrain | ||||
| 2.92 | −12 −22 8 | Thalamus | ||||
| 796 | 3.72 | −30 24 −2 | IFG | 47 | ||
| 3.64 | −32 26 32 | MFG | 9 | |||
| 3.56 | −52 18 36 | MFG | 9 | |||
| 3.46 | −44 12 26 | IFG | 9 | |||
| 3.34 | −42 2 28 | IFG | 9 | |||
| 3.32 | −32 16 10 | Insula | 13 | |||
| 3.32 | −28 24 10 | Insula | 13 | |||
| 3.06 | −32 8 32 | IFG | 9 | |||
| 2.99 | −40 14 8 | Insula | 13 | |||
| 2.93 | −42 8 18 | Insula | 13 | |||
| 2.92 | −32 20 18 | Insula | 13 | |||
| 2.90 | −42 20 22 | MFG | 46 | |||
| 2.84 | −44 28 32 | MFG | 9 | |||
| b. Amygdala Mask
| |||||
|---|---|---|---|---|---|
| Contrast | Cluster Extent Threshold | # Voxels | Peak T Value | Peak MNI Coordinates x, y, z | Anatomical Area(s) |
| (I-C, LE task)-(I-C, HE task) | 13 | 16 | 3.47 | 36 2 −24 | Right amygdala |
| cI-iI, LE task | 12 | 13 | 3.13 | 28 −2 −26 | Right amygdala |
Note. Contrasts were originally thresholded at p <.005, and then corrected for multiple comparisons using a cluster extent threshold as determined in AlphaSim (p <.05). Up to 20 local maxima are reported for each cluster. Description of the anatomical area of each of these sub-peaks is provided, as well as Brodmann area (when applicable).
I = incongruent; C = congruent; cI = incongruent trial preceded by incongruent trial; iI = incongruent trial preceded by incongruent trial; HE = high expectancy task; LE = low expectancy task; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; FG = frontal gyrus; IPL = inferior parietal lobule; STG = superior temporal gyrus; ITG = inferior temporal gyrus; MTG = middle temporal gyrus; SFG = superior frontal gyrus; SPL = superior parietal lobule.
The tasks were then directly compared. As expected, the HE task did not significantly activate any regions more strongly than the LE task. However, for (I-C, LE task – I-C, HE task), a large cluster of over 45,000 voxels was significant, and included many of the areas described above for the I-C, LE task contrast, including insula, dACC and cingulate gyrus (extending into SMA) and lateral PFC (BA 47 and 46) (Figure 4a, c, Table 2). The amygdala mask analysis showed a significant cluster of activation in the right amygdala (16 voxels), showing that incongruent event related activity in this region is stronger in the LE task compared to the HE task (Table 2).
Because anxiety was negatively correlated with behavioral adjustments in RT and accuracy on I trials in the HE task compared to the LE task, a multiple regression was performed on the contrast (I, HE task – I, LE task) and (I, LE task – I, HE task), with trait anxiety as a covariate of interest. There were no areas of significant activity.
Analysis 1: Trial-to-Trial Effects
For the trial-to-trial effects, we performed the contrasts listed above in the methods section. Four contrasts revealed significant activity. For cI-iI, HE task, there was activity in parahippocampal gyrus, superior temporal gyrus, middle temporal gyrus/angular gyrus, precuneus, medial frontal gyrus, posterior cingulate, and middle frontal gyrus (extending into BA 6, 8 and 9) (Table 2). Most of these regions are part of the default mode network (Buckner, Andrews-Hanna, & Schacter, 2008). If the default mode network is further deactivated on the iI trial as a result of implementation of control or task-related processing, this would result in the pattern we observed (ie, less of a deactivation of the default mode network on the cI trial) (McKiernan, Kaufman, Kucera-Thompson, & Binder, 2003). For the contrasts cI, LE task – cI, HE task, and iI, LE task – iI, HE task, there was activity in an extensive medial and lateral prefrontal network, including bilateral DLPFC and VLPFC, as well as dACC (Table 2). Lastly, there was significant right amygdala activity for the contrast cI-iI, LE task (Table 2). This shows that the greater incongruent-related amygdala activity that occurs in the LE task can be attributed to the high-conflict cI trial.
Analysis 2: Sustained Activity
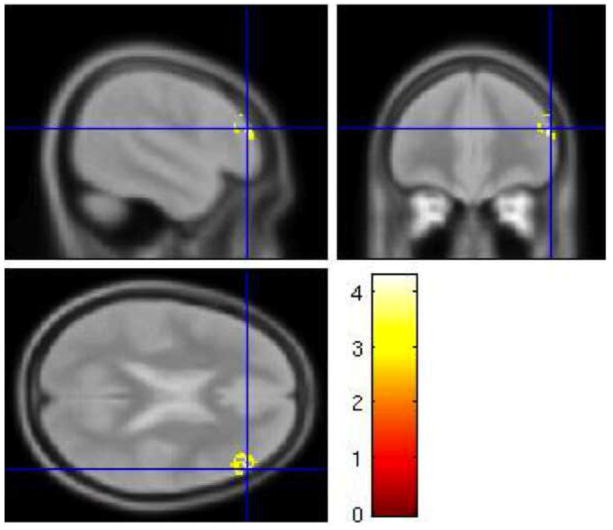
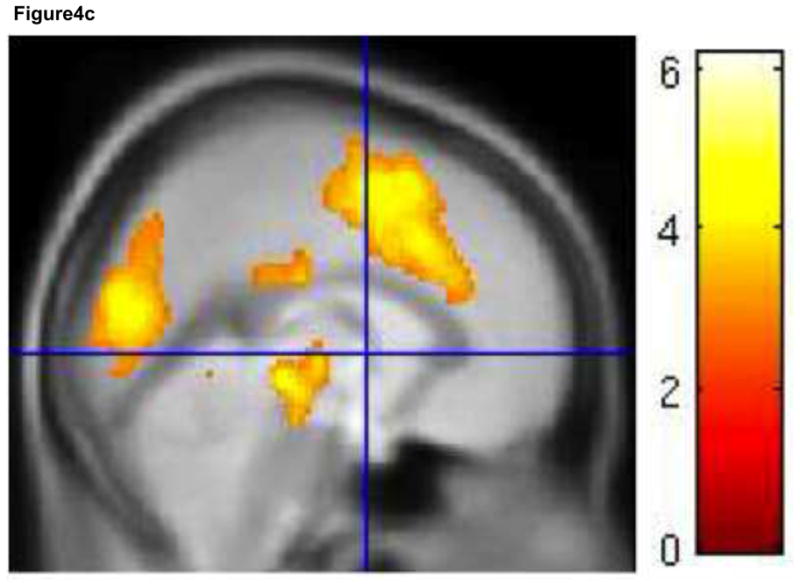
While the event-related analysis did not reveal any activation during specific trials of the HE task, significantly greater sustained activity in right DLPFC (BA 46) was observed during HE task performance compared to rest (periods during which subjects were instructed to rest, relax, and not think about the task) (contrast: (HE task-rest) – (LE task-rest)) (Figure 5). There were no significant areas of activation within the rACC mask. We also looked at the whole brain results for this contrast, and although they would not have survived correction for multiple comparisons, there were fairly large clusters of activity in right inferior and superior parietal lobule (366 voxels) and right fusiform gyrus (226 voxels) at p<.005, uncorrected (Table 3). There was no significant activity for the opposite contrast, (LE task-rest) – (HE task-rest) within the masks. There was also no sustained activity for this contrast when we looked at the whole brain at a liberal threshold (p<.005, uncorrected).
Figure 5.
Sustained activation. For the contrast (HE task rest) – (LE task-rest) there was greater sustained activity in right DLPFC (BA 46). This contrast was performed using a DLPFC (BA 9/46) mask. Results are displayed at p < .005, with a cluster extent threshold that corrects for multiple comparisons (p<.05) (see methods). HE = high expectancy task; LE = low expectancy task; DLPFC = dorsolateral prefrontal cortex; BA = brodmann area. Colorbar indicates T statistic.
Table 3.
fMRI Summary Data- Analysis 2: Sustained Activity
| a. DLPFC Mask
| ||||||
|---|---|---|---|---|---|---|
| Contrast | Cluster Extent Threshold | # Voxels | Peak T Value | Peak MNI Coordinates x, y, z | Anatomical Areas(s) | Brodmann Area(s) |
| (HE task- rest) – (LE task-rest) | 130 | 130 | 4.27 | 52 38 20 | MFG | 46 |
| 3.27 | 50 36 30 | MFG | 46 | |||
| 3.23 | 54 30 24 | MFG | 46 | |||
| 3.20 | 44 34 20 | MFG | 46 | |||
| b. Whole Brain Exploratory
| |||||
|---|---|---|---|---|---|
| Contrast | # Voxels | PeakT Value | Peak MNI Coordinates x, y, z | Anatomical Areas (s) | Brodmann Area(s) |
| (HE task-rest)–(LE task-rest) | 407 | 4.42 | 52 36 20 | MFG | 46 |
| 3.86 | 54 32 28 | MFG | 46 | ||
| 3.86 | 38 26 20 | Insula | 13 | ||
| 366 | 4.25 | 34 −60 50 | SPL | 7 | |
| 3.87 | 38 −56 38 | IPL | 40 | ||
| 3.09 | 26 −56 48 | SPL | 7 | ||
| 226 | 3.92 | 50 −46 −16 | Fusiform gyrus | 37 | |
| 3.67 | 64 −42 −10 | MTG | 21 | ||
| 3.44 | 60 −48 0 | MTG | 22 | ||
| 3.00 | 50 −44 −8 | MTG | 37 | ||
| 3.94 | 60 −58 2 | MTG | 21 | ||
| Positive Correlation with Trait Anxiety | |||||
| HE task-rest | 223 | 3.42 | 52 −8 56 | Precentral gyrus | 6 |
| 3.28 | 58 −6 48 | Precentral gyrus | 6 | ||
| 3.18 | 52 −26 60 | Postcentral gyrus | 1 | ||
| 3.12 | 50 −30 46 | IPL | 40 | ||
| 3.10 | 58 −14 52 | Postcentral gyrus | 3 | ||
| 2.87 | 52 −20 54 | Postcentral gyrus | 1 | ||
| 2.78 | 62 −2 38 | Precentral gyrus | 6 | ||
| (HE task-rest)–(LE task-rest) | 2,866 | 5.66 | 34 −16 72 | Precentral gyrus | 6 |
| 5.22 | 56 −8 42 | Precentral gyrus | 6 | ||
| 5.18 | 54 −18 52 | Postcentral gyrus | 3 | ||
| 4.97 | 36 −16 64 | Precentral gyrus | 6 | ||
| 4.66 | 48 −26 58 | Postcentral gyrus | 1 | ||
| 3.96 | 50 −8 24 | Precentral gyrus | 6 | ||
| 3.91 | 54 −2 30 | Precentral gyrus | 6 | ||
| 3.87 | 48 −12 40 | Precentral gyrus | 6 | ||
| 3.52 | 42 −42 58 | IPL | 40 | ||
| 3.52 | 54 −16 18 | Postcentral gyrus | 43 | ||
| 3.40 | 62 −14 16 | Transverse temporal gyrus | 42 | ||
| 3.29 | 46 −32 40 | Postcentral gyrus | 2 | ||
| 3.20 | 60 −2 24 | IPL | 6 | ||
| 3.13 | 44 −40 48 | Precentral gyrus | 40 | ||
| 2.98 | 40 −42 36 | Supramarginal gyrus | 40 | ||
| 2.79 | 46 −22 20 | Insula | 13 | ||
| 323 | 3.71 | −48 −16 34 | Precentral gyrus | 6 | |
| 3.68 | −44 −6 32 | Precentral gyrus | 6 | ||
| 3.15 | −46 −18 20 | Insula | 13 | ||
| 3.00 | −38 2 34 | Precentral gyrus | 6 | ||
| 165 | 3.44 | −42 −40 58 | IPL | 40 | |
| 3.34 | −48 −38 48 | IPL | 40 | ||
Note. Up to 20 local maxima are reported for each cluster. Description of the anatomical area of each of these sub-peaks is provided, as well as Brodmann area (when applicable).
a) Contrasts were originally thresholded at p <.005, and then corrected for multiple comparisons using a cluster extent threshold as determined in AlphaSim (p <.05). A DLPFC mask (BA 9 and BA 46) was created using the WFU Pickatlas (Maldjian JA et al., 2003).
b) For this exploratory whole brain analysis, a threshold of, p<.005, > 100 voxels (uncorrected) was used.
HE = high expectancy task; LE = low expectancy task; MFG = middle frontal gyrus; SPL = superior parietal lobule; IPL = inferior parietal lobule; MTG = middle temporal gyrus.
Because lower anxiety was behaviorally correlated with better performance on the HE task compared to the LE task, HE task-rest and (HE task-rest) – (LE task-rest) were correlated with trait anxiety. There was no significant activity in the masked DLPFC or rACC regions for either of these contrasts.
Analysis 3: Emotion and Conflict
Because cI neutral face trials caused the most behavioral interference, we focused our fMRI analysis on these trials, particularly on differences between activation on cI neutral face trials in the LE task (maximum behavioral interference), and cI neutral face trials in the HE task (same trial type as in the LE task, but much less behavioral interference). First, cI neutral face trials were compared to cI fearful face trials in both tasks separately. There was no greater activity for cI neutral face trials compared to cI fearful face trials in either of the two tasks (contrasts: cI neutral face – cI fearful face, HE task; cI neutral face – cI fearful face, LE task).
Second, the cI neutral face – cI fearful face contrast was directly compared between tasks. While the contrast (cI neutral face – cI fearful face, HE task) – (cI neutral face – cI fearful face, LE task) did not show any activity, the opposite contrast, (cI neutral face – cI fearful face, LE task) – (cI neutral face – cI fearful face, HE task) showed several areas that were active more for cI neutral face trials in the LE task. This included right and left inferior parietal lobule, insula, middle temporal gyrus, lateral prefrontal cortex (BA 9, 46, 47), caudate, and dACC (Table 4).
Table 4.
fMRI Summary Data- Analysis 3: Emotion and Conflict
| Contrast | Cluster Extent Threshold | # Voxels | Peak T Value | Peak MNI Coordinates x, y, z | Anatomical Area(s) | Brodmann Area(s) |
|---|---|---|---|---|---|---|
| (cI neutral face – cI fearful face, LE task) – (cI neutral face – cI fearful face, HE task) | 341 | 1,208 | 5.78 | 58 −40 44 | IPL | 40 |
| 4.79 | 54 −38 36 | IPL | 40 | |||
| 4.14 | 48 −34 32 | IPL | 40 | |||
| 4.10 | 52 −44 28 | IPL | 40 | |||
| 4.06 | 46 −48 60 | IPL | 40 | |||
| 4.05 | 54 −44 56 | IPL | 40 | |||
| 3.61 | 38 −54 64 | SPL | 7 | |||
| 685 | 5.40 | 56 14 6 | Precentral gyrus | 44 | ||
| 3.50 | 40 12 0 | Insula | 13 | |||
| 3.39 | 30 28 −10 | IFG | 47 | |||
| 3.30 | 42 4 4 | Insula | 13 | |||
| 3.05 | 34 18 −4 | IFG | 47 | |||
| 2.82 | 22 28 −12 | MFG | 11 | |||
| 482 | 4.24 | 54 6 42 | MTG | 21 | ||
| 3.82 | 46 4 42 | MFG | 9 | |||
| 3.63 | 46 2 56 | MFG | 6 | |||
| 3.15 | 40 14 34 | MFG | 9 | |||
| 3.03 | 34 6 34 | IFG | 9 | |||
| 2.86 | 38 −2 32 | Precentral gyrus | 6 | |||
| 2.75 | 46 18 38 | Precentral gyrus | 9 | |||
| 417 | 3.94 | 8 14 4 | Caudate | |||
| 3.52 | 16 16 2 | Caudate | ||||
| 3.38 | 12 8 14 | Caudate | ||||
| 3.28 | −6 4 4 | Caudate | ||||
| 3.21 | 16 18 10 | Caudate | ||||
| 2.82 | 20 0 10 | Lentiform nucleus | ||||
| 470 | 3.89 | 8 24 46 | Cingulate gyrus | 32 | ||
| 3.76 | −12 14 42 | Cingulate gyrus | 32 | |||
| 3.38 | 4 28 54 | SFG | 8 | |||
| 2.88 | −6 10 34 | Cingulate gyrus | 24 | |||
| 549 | 3.71 | −42 −40 36 | Supramarginal gyrus | 40 | ||
| 3.63 | −56 −50 46 | IPL | 40 | |||
| 3.47 | −46 −44 42 | Cerebellum | ||||
| 3.45 | −48 −42 50 | IPL | 40 | |||
| 3.21 | −52 −48 54 | IPL | 40 | |||
| 3.08 | −54 −46 36 | Supramarginal gyrus | 40 | |||
| 513 | 3.68 | 38 32 28 | MFG | |||
| 3.51 | 40 48 4 | IFG | 10 | |||
| 3.42 | 34 32 20 | IFG | 46 | |||
| 3.41 | 32 26 26 | MFG | ||||
| 3.20 | 44 40 24 | MFG | 46 | |||
| 3.16 | 42 50 16 | MFG | 10 | |||
| 3.03 | 46 40 8 | IFG | 46 | |||
| 2.80 | 40 42 −4 | MFG | 47 | |||
| 396 | 3.66 | −44 12 12 | Precentral gyrus | 44 | ||
| 3.48 | −36 20 −6 | IFG | 47 | |||
| 3.45 | −54 0 10 | Precentral gyrus | 6 | |||
| 3.44 | −46 6 4 | Insula | 13 | |||
| 3.44 | −60 8 10 | Precentral gyrus | 44 | |||
| 3.01 | −36 12 2 | Insula | 13 | |||
| 2.71 | −34 10 14 | Insula | 13 | |||
| (cI neutral face, LE task) – (cI neutral face, HE task) | 408 | 19,197 | 6.72 | 42 16 4 | Insula | 13 |
| 6.65 | 58 −44 48 | IPL | 40 | |||
| 5.93 | 30 54 −10 | MFG | 10 | |||
| 5.81 | 40 50 −10 | MFG | 11 | |||
| 5.77 | −30 22 10 | Insula | 13 | |||
| 5.68 | −30 14 12 | Insula | 13 | |||
| 5.53 | 12 6 14 | Caudate | ||||
| 5.35 | 48 −58 52 | IPL | 40 | |||
| 5.31 | −14 16 12 | Caudate | ||||
| 5.27 | −40 10 10 | Insula | 13 | |||
| 5.16 | 34 24 8 | IFG | 45 | |||
| 5.06 | −8 6 6 | Caudate | ||||
| 5.01 | 46 −48 60 | IPL | 40 | |||
| 4.95 | −38 16 4 | Insula | 13 | |||
| 4.93 | 34 46 −14 | SFG | 11 | |||
| 4.87 | 60 −42 34 | Supramarginal gyrus | 40 | |||
| 4.85 | 14 14 8 | Caudate | ||||
| 4.85 | 18 14 −8 | Lentiform nucleus | ||||
| 4.84 | 44 48 18 | MFG | 10 | |||
| 4.83 | 4 −14 2 | Thalamus | ||||
| 2,403 | 5.81 | −54 −48 48 | IPL | 40 | ||
| 5.37 | −58 −54 36 | Supramarginal gyrus | 40 | |||
| 4.79 | −46 −56 56 | IPL | 40 | |||
| 4.50 | −52 −60 44 | IPL | 40 | |||
| 4.28 | −16 −64 66 | SPL | 7 | |||
| 4.12 | −64 −42 16 | STG | 22 | |||
| 4.05 | −46 −46 36 | Supramarginal gyrus | 40 | |||
| 3.62 | −42 −46 46 | IPL | 40 | |||
| 3.59 | −24 −64 62 | SPL | 7 | |||
| 3.53 | −60 −48 4 | MTG | 21 | |||
| 3.51 | −58 −36 44 | IPL | 40 | |||
| 3.28 | −32 −50 50 | SPL | 7 | |||
| 3.21 | −50 −46 8 | MTG | 21 | |||
| 3.16 | −30 −54 40 | SPL | 7 | |||
| 3.07 | −54 −54 0 | MTG | 37 | |||
| 2,632 | 5.56 | 8 24 40 | Cingulate gyrus | 32 | ||
| 5.19 | 10 16 44 | Cingulate gyrus | 32 | |||
| 4.46 | −10 10 42 | Cingulate gyrus | 32 | |||
| 4.24 | 4 28 54 | SFG | 8 | |||
| 3.81 | 8 2 60 | Medial FG | 6 | |||
| 3.56 | −6 14 50 | Medial FG | 6 | |||
| 3.55 | 12 22 58 | SFG | 6 | |||
| 3.53 | 6 10 28 | Anterior cingulate | 33 | |||
| 3.21 | −4 10 28 | Anterior cingulate | 33 | |||
| 3.20 | −14 32 34 | Medial FG | 9 | |||
| 3.09 | 10 8 52 | Medial FG | 32 | |||
| 2.94 | −6 30 28 | Anterior cingulate | 32 | |||
| 522 | 4.16 | −4 −26 30 | Cingulate gyrus | 23 | ||
| 4.13 | 8 −26 26 | Cingulate gyrus | 23 | |||
| 3.70 | 6 −26 46 | Paracentral lobule | 31 | |||
| 3.20 | 12 −20 48 | Cingulate gyrus | 24 | |||
| 2.98 | 16 −30 48 | Cingulate gyrus | 31 | |||
| 413 | 3.55 | 10 −76 40 | Precuneus | 7 | ||
| 3.53 | −2 −70 42 | Precuneus | 7 | |||
| 2.77 | −10 −58 34 | Precuneus | 31 | |||
| Negative Correlation with Trait Anxiety cI neutral face – baseline, HE task | 463 | 560 | 4.32 | 50 −32 −12 | MTG | 20 |
| 3.64 | 66 −20 −16 | MTG | 21 | |||
| 3.64 | 62 −42 −10 | MTG | 21 | |||
| 3.34 | 64 −12 −20 | ITG | 21 | |||
| 3.27 | 58 −28 −16 | MTG | 21 | |||
| 2.89 | 62 −4 −24 | MTG | 21 | |||
| 773 | 4.03 | −32 22 −16 | IFG | 47 | ||
| 3.85 | −42 20 0 | IFG | 47 | |||
| 3.70 | −42 24 −8 | IFG | 47 | |||
| 3.38 | −46 12 −10 | STG | 38 |
Note. Contrasts were originally thresholded at p <.005, and then corrected for multiple comparisons using a cluster extent threshold as determined in AlphaSim (p <.05). Up to 20 local maxima are reported for each cluster. Description of the anatomical area of each of these sub-peaks is provided, as well as Brodmann area (when applicable).
HE = high expectancy task; LE = low expectancy task; cI = incongruent trial preceded by congruent trial; IPL = inferior parietal lobe; SPL = superior parietal lobule; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; MTG = medial temporal gyrus; SFG = superior frontal gyrus; STG = superior temporal gyrus; FG = frontal gyrus.
Lastly, cI neutral face trials for the HE and LE tasks were directly compared. Once again, there were no regions that were more active in the HE task compared to the LE task (contrast: (cI neutral face, HE task) – (cI neutral face, LE task)). However, for the opposite contrast, (cI neutral face, LE task) – (cI neutral face, HE task) there was activity in the dACC, insula, inferior parietal lobule, right lateral OFC/BA 11, and visual processing regions (Table 4).
Three contrasts were correlated with trait anxiety: (cI neutral face, HE task) – (cI neutral face, LE task), (cI neutral face, LE task) – (cI neutral face, HE task), and cI neutral face – baseline, HE task. Two significant clusters were negatively correlated with trait anxiety for the cI neutral face – baseline, HE contrast: a 560-voxel cluster in right middle temporal gyrus, and a 773-voxel cluster in left inferior frontal gyrus, extending into BA 47, lateral OFC and anterior insula. These two regions are thus more active in subjects with low anxiety during cI neutral face trials in the HE task (Figure 6, Table 4).
Figure 6.
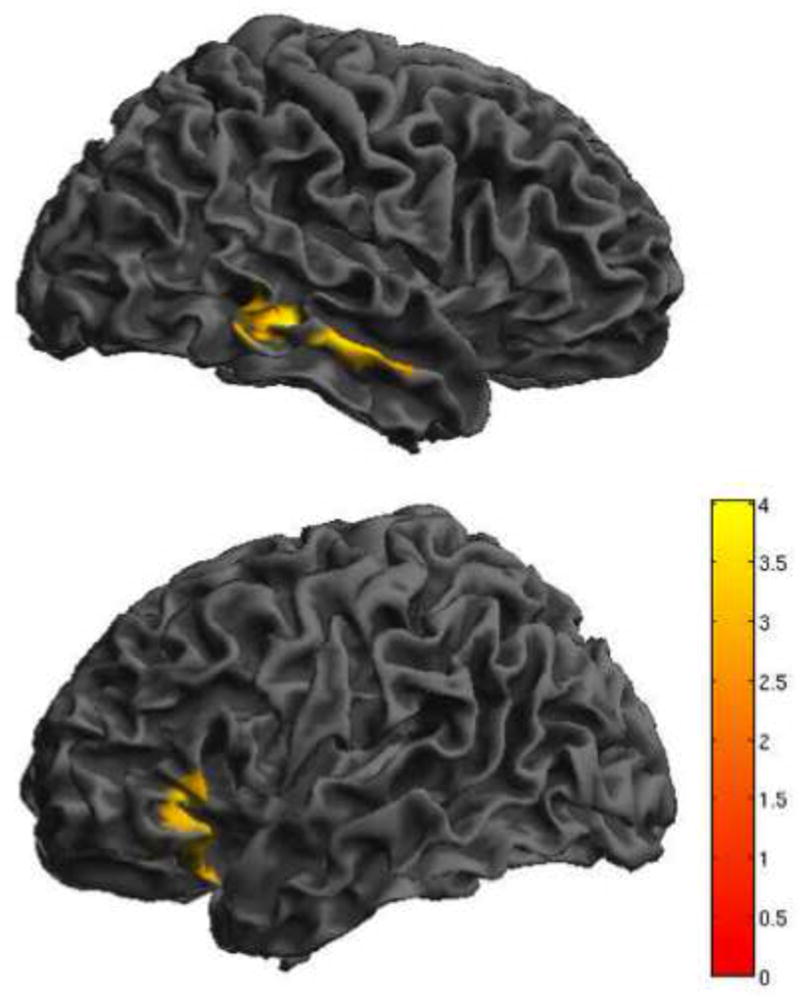
Individual differences in activation. Activity for cI neutral face trials, HE task was negatively correlated with trait anxiety in two regions: right middle temporal gyrus and a left lateralized cluster that spans inferior frontal gyrus/ BA 47, lateral OFC and the anterior insula.
(contrast: cI neutral face, HE task – baseline). Results are displayed at p < .005, with a cluster extent threshold that corrects for multiple comparisons (p<.05) (see methods). HE = high expectancy task; cI = incongruent trial preceded by congruent trial; BA = brodmann area; OFC = orbitofrontal cortex. Colorbar indicates Z-statistic.
3. Discussion
In summary, increasing the proportion of I trials in an emotional facial Stroop task reduces behavioral interference effects in both RT and accuracy. Incongruent trials in a LE task are characterized by event related activation of an extensive medial and lateral prefrontal cognitive control network previously associated with conflict monitoring and resolution, as well as the right amygdala. Incongruent trials in a HE task did not engage these areas on a trial-by-trial basis. The HE task was instead characterized by increased sustained activity, most notably in right DLPFC. Individual differences in trait anxiety had an effect on behavioral performance for the cI neutral face-fearful word trials. For RT, high trait anxiety was negatively correlated with speeding on the cI neutral face trial in the HE task compared to the LE task. In other words, subjects lower in anxiety made a better RT adjustment on the cI neutral face trial due to increased expectancy for I trials. High anxiety was negatively correlated with accuracy on the cI neutral face trial in the HE task. There were no differences in sustained activity that correlated with trait anxiety. However, for cI neutral face trials in the HE task, low anxiety was correlated with activity in two areas: right middle temporal gyrus and a large cluster that spanned left VLPFC, anterior insula, and lateral OFC.
This experiment is the first to demonstrate that an increase in proportion of I trials reduces behavioral interference effects in an emotional facial Stroop task. There is evidence that this particular emotional facial Stroop task is more difficult than a comparable non-emotional version (Krug & Carter, 2010), so it is especially noteworthy that proportion changes result in the predicted adjustments in cognitive control. In order to ascertain enough I trials for additional analyses, the proportion differences between the two tasks were quite subtle (65/35) compared to other studies (for example, in Carter et al. (2000), 80/20). This provides good evidence that the effects of proportion manipulation in emotional facial Stroop tasks are quite robust. Because subjects in this experiment only performed an emotional version of the facial Stroop task, this precludes direct comparisons of the magnitude of this behavioral interference reduction (and associated neural changes) in emotional versus non-emotional tasks. Future studies, particularly those in which the same subjects also perform a non-emotional comparison task (as in Egner et al. (2008) and Krug & Carter (2010)) will allow for the two tasks to be directly compared in order to more completely characterize the effects of emotion on proportion-dependent adjustments in cognitive control.
As indicated above, subjects were not told of the proportion manipulation, and were given no indication that there were any differences in the tasks they were performing. No subjects spontaneously mentioned the proportion manipulation, and even when explicitly asked, no subjects reported noticing the changes in proportion of I trials. Thus, although we use the word “expectancy” in reference to the likelihood of I trials, this does not imply that the subjects were consciously aware that they were encountering more or less I trials, and any changes in behavior and cognitive control most likely occurred subconsciously. It is not surprising that behavioral changes were observed despite lack of awareness of the proportion manipulation, as behavioral studies have shown that even when the irrelevant stimulus is masked so that it is not visible, increased proportion of I trials still causes a reduction in interference (Klapp, 2007).
Our finding of sustained activity in right DLPFC in the HE task (and absence of event-related activity in this region) suggests that proportion manipulation effects are possibly governed by a different control mechanism than the trial-to-trial activations observed in LE tasks that account for previous trial congruency effects. Our results fit well with the DMC theory (Braver et al., 2007), as well as De Pisapia and Braver (2006), who found transient dACC activity and lateral PFC activity in a LE Stroop task, and sustained lateral PFC activity in a HE Stroop task. Notably, the sustained activity observed in this experiment was also right-lateralized, although more posterior and dorsal compared to the activation observed by De Pisapia and Braver (2006). Our results are also consistent with reports that sustained attention may engage right-lateralized prefrontal regions (Pardo, Fox, & Raichle, 1991; Posner & Petersen, 1990). Our results are not in accordance with Dosenbach et al. (2008; 2007; 2006) as we did not find any evidence of sustained activity in dACC.
The presence of sustained fusiform activity suggests that DLPFC could be working with fusiform gyrus in a top-down manner to increase task-relevant face processing, thus reducing behavioral interference effects (Egner & Hirsch, 2005). Our fusiform cluster, though sizeable, did not meet cluster level thresholds for significance. Future experiments should investigate this mechanism more specifically, perhaps through the use of a face-processing localizer task (to create a face-processing ROI) and functional connectivity analyses, which may provide more power to discern these interactions.
Behavioral results show that there are strong, significant trial-to-trial adjustments (conflict adaptation) occurring in the LE task. However, there is also a strong trend toward significance (p-values ranging from .054–.065) for trial-to-trial adjustments in the HE task, despite the overall reduction in interference and change in neural activity. This suggests that the cognitive boost from sustained control in the HE task serves to reduce the interference effects, but may not completely abolish the dynamic adjustments that are still occurring on a trial-by-trial basis. This supports the hypothesis that proactive and reactive control processes are complementary and that both contribute, in parallel, to supporting task performance during conflict eliciting tasks. It is important to note that while our basic interference effects (I vs. C) were in the hypothesized direction, the previous × current trial ANOVA’s for RT were driven by control related adjustments in congruent trials (iC slower than cC) and not in incongruent trials (cI slower than iI). We are not certain why these effects were not present. Consequently, neuroimaging results reported from cI vs. iI contrasts must be interpreted with caution as these two trial types were not associated with the predicted control related changes in RT.
The results of this experiment do not provide strong evidence that variation in trait anxiety in healthy volunteers is associated with an overall impairment in interference reduction in HE conditions, or disrupted recruitment of sustained control. Instead, higher trait anxiety is most strongly associated with impairment on trials characterized by high conflict and an emotional, task-irrelevant stimulus (cI neutral face-fearful word trial). This impairment persists, even when I trials are more commonly encountered. On this particular trial type, high anxiety is correlated with decreased recruitment of left IFG/anterior insula/lateral OFC.
Left IFG is often activated during trials in working memory tasks that invoke proactive interference (Badre & Wagner, 2005; D’Esposito, Postle, Jonides, & Smith, 1999; Jonides & Nee, 2006; Jonides, Smith, Marshuetz, Koeppe, & Reuter-Lorenz, 1998). Patient studies suggest that this region is involved specifically in proactive interference resolution (Hamilton & Martin, 2005; Thompson-Schill et al., 2002). Jonides & Nee (2006) suggest that left IFG determines the context in which a probe originally appeared, so a subject can determine if the probe appeared in the current trial (positive response) or a previous trial (negative response). The left IFG could be serving a similar purpose in this case, by assigning the “fearful” stimulus to the “irrelevant” context, since it is occurring in the word dimension, thus helping subjects to resist the automatic urge to respond to the fearful word on that particular trial.
The anterior insula is active in many different cognitive control tasks, including working memory tasks, go/no-go task, stimulus response compatibility task, and the Flanker task (Wager & Feldman Barrett, 2004; Wager et al., 2005). In a Flanker task, more activity in anterior insula is associated with less behavioral interference in both adults and children (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002). Shafritz, Collins, & Blumberg (2006) found activity in this region in an emotional go/no-go task, specifically on trials where subjects were required to inhibit a response to an emotional face. They suggest that the anterior insula incorporates emotional information when deciding whether to respond to a stimulus or withhold responding.
Lastly, both anterior insula and lateral OFC are associated with resolution of proactive interference caused by emotional information (Levens & Phelps, 2008). Both of these regions could act together to integrate emotional information into a plan of action (for discussion, see (Levens & Phelps, 2008; Wager & Feldman Barrett, 2004)). Our results support these theories. Low anxiety, which is associated with better performance on cI neutral face trials in the HE task, could be attributed to better activation of anterior insula and lateral OFC, which helps with the decision to withhold responding to the “fearful” word in the context of that particular trial.
Because the correlation of activity in these regions with trait anxiety involved a contrast with baseline (cI neutral face – baseline, HE task) there is the possibility that this activation could represent non specific task processing that happens to be associated with trait anxiety. To exclude this less interesting alternative, in a follow-up analysis we performed the same correlation with the following contrasts: cI fearful – baseline, HE task, cI fearful – baseline, LE task, and cI neutral face-baseline, LE task. For all three contrasts, there were no significant clusters of activation. We can conclude with reasonable certainty that the activations that were negatively correlated with trait anxiety for the cI neutral face – baseline, HE task contrast were specific to that combination of trial type (cI neutral face fearful word) and task context (high expectancy for incongruent trials).
While we did find that high anxiety was associated with poor performance on the cI neutral face-fearful word trial in the HE task, it was somewhat unexpected to find that trait anxiety had no effect on behavioral performance in the LE task. We had previously shown, in a LE version of the emotional facial Stroop task, that subjects low in anxiety were not impaired on the cI neutral face trial, while subjects high in anxiety did show RT slowing (Krug & Carter, 2010). This may have to do with differences in how subjects responded to the task instructions. In this experiment, subjects were told to perform quickly and accurately, while in the previous experiment, speed was heavily emphasized. In the previous experiment, the high anxiety group were (insignificantly) more accurate than the low anxiety group on the cI neutral face-fearful word trials (low anxiety= 90.8% accuracy, high anxiety= 93.7% accuracy) so we cannot exclude the possibility that strategic response to the speed instructions given in Krug & Carter (2010) may have differed as a function of trait anxiety. When speed is not emphasized, as in the current experiment, differences in behavioral performance as a function of trait anxiety may only be evident in HE conditions.
While it is quite apparent that the cI neutral-face fearful-word trial is more difficult for individuals with higher levels of anxiety, the interaction of task instructions and expectancy conditions could also bring out different neural mechanisms underlying these behavioral differences in performance. Under speed emphasis and LE conditions, impairment in disengagement from and over-processing of the task-irrelevant “fearful” word may have produced the pattern of behavioral and neural results (notably, enhanced dACC activity and lack of rACC activity) in the high anxiety group in our previous experiment (Krug & Carter, 2010). However, under speed and accuracy instructions and a HE context, differences in the implementation of more complex task integration and control-related processes mediated by left VLPFC, lateral OFC and anterior insula may underlie the observed behavioral effects that vary as a function of trait anxiety. A future avenue of research would be to investigate interactions between behavioral performance and trait anxiety as a function of task instructions and speed-accuracy trade-off.
It is important to note that, despite the changes in behavior and neural activity that significantly correlated with trait anxiety, our subjects were not from a clinical population. While we did not find strong evidence for differences in sustained control as a function of trait anxiety, this does not necessarily preclude our original hypothesis, that high anxiety is characterized by impairment in sustained control. This hypothesis needs to be tested again using more difficult cognitive control tasks, and in subjects with anxiety disorders. An additional improvement would be to use a mixed blocked/event-related design (Visscher et al., 2003), which is optimal for separating the fMRI signal into sustained and transient activity. In this experiment, the total fMRI signal was modeled in separate event-related and sustained analyses, leaving open the possibility that some transient activity could have contributed to the sustained activity results, and vice-versa.
In this experiment we did not find any evidence of rACC activity, in either the whole group data or as a function of individual differences in trait anxiety. In our previous experiment, we found that subjects low in anxiety activated rACC, while subjects high in anxiety did not (Krug & Carter, 2010). In the present experiment we had a much higher number of subjects (42, compared to 30 in Krug & Carter), however we did not have as many individuals with high scores on the trait anxiety scale. The average trait anxiety score is 35 and 39, for working adults and undergraduate students, respectively (Spielberger, 1983). In Krug & Carter (2010), 8 out of 30 subjects had a score above 35 (27%), while 2 of those subjects (7%) scored above 40. In this experiment, only 6 out of 42 subjects (14%) had a score above 35, and only 1 subject had a score above 40. Behavioral studies investigating attention to threat words indicate that individual differences in trait anxiety have the greatest effects on performance when trait anxiety scores are above 40 (Broadbent & Broadbent, 1988). If we had more individuals with higher trait anxiety, we may have been able to detect rACC as a function of individual differences in anxiety, as other studies have consistently shown an association between high anxiety and less activity in rACC (Bishop et al., 2004; Etkin et al., 2010; Mathews et al., 2004; Shin et al., 2001). Additionally, as mentioned above, differences in task instructions between the two experiments may have affected individual differences-related activation of rACC.
It is notable that we did observe amygdala activity in the LE task. In our previous study we did not find any congruency-related amygdala activation (Krug & Carter, 2010). However, the larger subject numbers may have increased sensitivity for finding conflict-related amygdala activity. We found increased right amygdala activity for incongruent trials (I-C, LE task – I-C, HE task), and, specifically, cI trials (cI-iI, LE task). This is in accordance with Etkin et al. (2006), who maintain that the amygdala may act as an emotional conflict detector. The strong activation of dACC and LPFC in our LE task, in combination with right amygdala, shows that instead of recruiting a separate emotion conflict/control network, that the typical “cognitive control” network is activated, but with the recruitment of additional emotion-processing regions such as the amygdala, possibly to aid in conflict detection/monitoring in the context of emotional stimuli.
It is important to note that the effects of emotion on conflict processing and control-related adjustments may be highly dependent upon the type of emotional task that is used. For example, Etkin et al. (2006) and Egner et al. (2008) used fearful and happy faces, creating conflict between stimulus dimensions of opposing valence. They showed more pronounced trial-to-trial adjustment effects and emotion-control related activity in rACC compared to the current task. When an emotional stimulus is used as a distracter (presented in between Stroop trials), conflict adaptation effects are reduced (Padmala, Bauer, & Pessoa, 2011). However, when emotional content is present in the attended stimulus, but irrelevant to the task at hand (a non-emotion-related task is required), congruency interference effects are reduced and emotion-processing regions such as amygdala and ventral ACC are engaged (Kanske & Kotz, 2011a, 2011b). Even reward associations can affect performance differentially, depending upon which aspect of the stimulus is associated with reward; when the attended ink color is associated with reward, Stroop task performance is improved, and when the task-irrelevant word meaning is associated with a “reward color”, interference effects are increased (Krebs, Boehler, Egner, & Woldorff, 2011). Future experiments should investigate proportion manipulations in these types of tasks and contexts to get a complete picture of how emotion affects implementation of cognitive control.
In conclusion, increasing the proportion of I trials in an emotional facial Stroop task reduces congruency interference effects in both RT and accuracy. The increase in expectancy for I trials is characterized by a switch from event-related engagement of right amygdala and a medial and lateral cognitive control network to sustained engagement of right DLPFC. Lastly, individual differences in trait anxiety contribute to changes in behavior and brain activity. Low anxiety is correlated with better performance in the HE condition, particularly during trials of high conflict and in which a task-irrelevant emotional stimulus must be ignored. These differences are most likely mediated by better recruitment of a VLPFC/anterior insula/lateral OFC region in low anxious subjects, which may help them to integrate the relevance of the emotional content of the stimulus with decision making and cognitive control.
4. Methods and Materials
Subjects
Subjects were recruited from the local university population and community. Potential subjects were screened over the phone and were excluded from the study if they were not right-handed, had a psychiatric or neurological history, were taking psychoactive medications, or had MRI contraindications. Due to the verbal nature of the Stroop interference effect, only fluent English speakers who had learned to speak English before the age of 6 were invited to participate. Of the forty-five participants scanned one subject was excluded for movement exceeding 4 mm and two subjects were excluded for structural abnormalities. For the remaining 42 subjects, 26 were female and16 were male, M age = 23.2 (SD = 4.6), range = 18–37. Participants were paid $30 per hour for the length of the testing session, which included consent, questionnaires, fMRI scanning, and debriefing. All subjects provided written informed consent according to the local Institutional Review Board-approved protocol.
Emotional Facial Stroop Task
Each subject performed an emotional facial Stroop task while undergoing fMRI scanning. Each trial consisted of a word and face stimulus. Trial onset began with the presentation of the word “neutral” or “fearful” (100 ms), followed by the simultaneous presentation of the word and a response congruent or response incongruent neutral or fearful face (900 ms) (Figure 1). Trials were separated by a fixation that ranged from 2000 ms to 13000 ms (mean fixation: 4000 ms).
The word was always presented in the color red, with an Arial size 32 font, and appeared in the center of the screen. When the word and face appeared together, the word appeared on top of the face, with the face positioned so the word did not obstruct the eyes of the face. Face stimuli were from the NimStim set (available at www.macbrain.org3). All face stimuli were converted to grayscale using Adobe photoshop software (Adobe Photoshop CS2 version 9.0.2). Fearful and neutral faces from the following individuals were used in this experiment: 3, 5, 7, 8, 9, 10, 11, 12, 13, 14, 17, 18, 19, 20, 21, 23, 26, 27, 28, 29, 30, 31, 32, 33, 34, 36, 37, 38, 39, 40, 41, 42, and 43. The closed mouth fearful and neutral faces were used for 7, 8, 20, 41, and 43, while the open mouth fearful and neutral faces were selected for the remaining individuals.
Two versions of the emotional facial Stroop task were used. In the HE task, 65% of trials were incongruent (neutral face-fearful word or fearful face-neutral word), while the remaining 35% of trials were congruent (neutral face-neutral word or fearful face-fearful word). In the LE task only 35% of trials were incongruent and 65% were congruent. For each task block, subjects received a pseudorandom presentation of trials. While the face stimulus presented at each trial was random, trial congruency was fixed such that the proper proportions of I trials were maintained for each block. The sequence of trials and length between trials was determined using the OptSeq2 program (http://surfer.nmr.mgh.harvard.edu/optseq/) to maximize the ability to detect signal differences between I and C trials.
Additionally, for both the HE task and the LE task the trial sequence was designed to produce sufficient cI trials; 20 per block, half of which were cI neutral face fearful word trials. Each subject was presented with a total of 40 cI neutral face-fearful word trials in the HE task and 40 cI neutral face-fearful word trials in the LE task.
A rest period was also included at the end of each task block. After all task trials were completed, a screen would appear notifying the participant that the task block was finished and no more face/word stimuli would be appearing. A fixation cross would then appear on the center of the screen for 30s. Because there were four task blocks for both the HE task and the LE task, there were four rest periods for each of the two tasks.
Trait Anxiety Assessment
All participants completed the Trait Anxiety Inventory (STAI) prior to the scanning session (Spielberger, 1983). Mean trait anxiety score was 30.2 (SD = 5.6) with a range of 21–47.
Procedure
Both versions of the emotional facial Stroop task were performed in the scanner. The task stimuli were presented using Eprime version 1.2.1.884 (Psychology Software Tools, Inc., Pittsburgh, Pennsylvania; http://www.pstnet.com) on a standard Dell computer connected to a projector, which displayed the stimulus onto a screen located at the foot of the scanner bed. Subjects viewed the stimulus via an adjustable mirror attached to the head coil.
Subjects were instructed to respond via button press (right index or middle finger) to indicate whether the face stimulus was neutral or fearful in emotion, while ignoring the word. They were advised to respond as quickly as possible without making too many errors.
Each subject performed 4 blocks of 100 trials for each of the two versions of the task. Because each block had a long duration of nearly 9 minutes, subjects performed 4 blocks of one task, and then were removed from the scanner for a brief 10–15 minute break. They then returned to the scanner to complete 4 blocks of the second version of the task. Order of tasks (HE task blocks first vs. LE task blocks first) and response mappings (index finger = neutral, middle finger = fearful vs. index finger = fearful, middle finger = neutral) were randomly assigned at the onset of each subject’s testing session. Subjects were not informed of the trial proportion manipulation, and were given no information regarding differences in the task performed before and after the break. No subjects reported noticing the proportion manipulation, neither when probed in a post-scan written questionnaire, nor when asked by the experimenter upon completion of the experiment. During rest periods, subjects were instructed to relax, but to remain awake with attention focused on the fixation cross.
Image Acquisition
Functional imaging was performed on a 3 Tesla Siemens Trio MRI system. Each of the two sessions included four runs of functional imaging. A T2*-weighted EPI sequence was used to acquire thirty-four oblique axial slices with a thickness of 3.2 mm (repetition time (TR) = 2000 ms, echo time (TE) = 27 ms, flip angle = 80°, field of view = 231 mm, matrix = 72 × .72) per TR. Slices were acquired parallel to the AC-PC line.
Image Preprocessing
Functional data was preprocessed using SPM8 software (Functional Imaging Laboratory, University College London, London, United Kingdom, http://www.fil.ion.ucl.ac.uk/spm). Preprocessing included the following steps: slice timing correction (to the first slice acquired), realignment (to the mean functional image, using a 2nd degree B-spline interpolation), normalization (to the EPI template), and smoothing (using an 8mm FHWH Gaussian kernel). All preprocessed data were resampled to 2 × 2 × 2 mm voxels, and reported in MNI sterotaxic space. Because each subject was removed from the scanner between session 1 and session 2, it is likely that head position in the scanner was slightly different. Consequently, realignment and normalization steps were performed separately for session 1 and session 2. A 128-second high-pass filter was applied to the functional data to removed low frequency fluctuations in signal.
fMRI Analysis
Preprocessed functional data were analyzed in SPM8 using a random effects general linear model (GLM).
Analysis 1: Interference and Trial-to-Trial Effects
The purpose of the first analysis was to compare conflict and control related activity for the HE and LE tasks. For our first GLM, I and C trials for each of the two tasks were entered as covariates and convolved with the canonical hemodynamic response function (HRF) for each individual subject. Previous trial congruency for I trials was also taken into account, and for relevant contrasts, both cI and iI trial events were included in the “I” variable. Covariates of interest were: 1) cI, HE task, 2) iI, HE task, 3) C, HE task, 4) cI, LE task, 5) iI, LE task, and 6) C, LE task. Errors, trials following errors, and trials in which the subject did not respond were modeled separately and were not included in the contrasts of interest. Beta estimates for the covariates were used to create the following contrasts for each subject: 1) I-C, HE task, 2) I-C, LE task, 3) (I-C, HE task) – (I-C, LE task), 4) (I-C, LE task) – (I-C, HE task), 5) I, HE task – I, LE task, 6) I, LE task – I, HE task, 7) cI-iI, HE task, 8) iI-cI, HE task, 9) cI-iI, LE task, 10) iI-cI, LE task, 11) (cI-iI, HE task) – (cI –iI, LE task), 12) (cI-iI, LE task) – (cI-iI, HE task), 13) (iI-cI, HE task) – (iI-cI, LE task), 14) (iI-cI, LE task) – (iI-cI, HE task), 15) cI, HE task – cI, LE task, 16) cI, LE task – cI, HE task, 17) iI, HE task – iI, LE task, 18) iI, LE task – iI, HE task.
Analysis 2: Sustained Activity
This analysis was performed to look at sustained task-related activity compared to sustained activity during rest periods. Due to protocol error, 6 subjects were not presented with the rest periods after each task block. Consequently, only the 36 subjects who received task blocks with rest periods were included in this analysis. For each task block, a “task” and “rest” covariate was created by convolving the canonical hemodynamic response function with a 20s duration. For the task covariate, onset was locked to a specified timepoint, and not onset of a trial. For each task block, the task covariate included three timepoints: sustained activity in the beginning of the task (20s into the task block), sustained activity in the middle of the task (240s into the task block) and sustained activity at the end of the task (440 seconds into the task block). Only one timepoint was included in the rest covariate of each block, because there was only one rest session per block. Task and rest covariates for each of the four blocks per task were combined to create the following covariates: 1) HE task, 2) HE rest, 3) LE task, and 4) LE rest. The following contrasts were created for each subject: 1) HE task-rest 2) LE task-rest, 3) (HE task-rest) – (LE task-rest), and 4) (LE task-rest) – (HE task-rest).
Analysis 3: Emotion and Conflict
The goal of the third analysis was to investigate differences in brain activity for the HE and LE tasks, with a focus on the cI neutral face-fearful word trial. The following trial types were modeled as covariates of interest: 1) cI neutral face, HE task, 2) cI neutral face, LE task, 3) cI fearful face, HE task, and 4) cI fearful face, LE task. Errors, trials following errors, trials in which the subject did not respond, iI trials, and C trials were modeled separately but not included in the contrasts of interest. Beta estimations were used to create the following contrasts for each individual: 1) cI neutral face – cI fearful face, HE task, 2) cI neutral face – cI fearful face, LE task, 3) (cI neutral face – cI fearful face, HE task) – (cI neutral face – cI fearful face, LE task), 4) (cI neutral face – cI fearful face, LE task) – (cI neutral face – cI fearful face, HE task) 5) cI neutral face, HE task – cI neutral face, LE task, 6) cI neutral face, LE task – cI neutral face, HE task, 7) cI neutral face – baseline, HE task, and 8) cI neutral face – baseline, LE task.
Group Analyses
For the three analyses described above, individual contrasts were submitted to group level t-tests. Whole-brain results were thresholded at the voxel level at p<.005, and then corrected for multiple comparisons (p<.05, FWE) using a cluster extent threshold determined using AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). In light of individual differences in behavioral performance as a function of trait anxiety, some of the contrasts described above were submitted to a multiple regression analysis at the group level, with trait anxiety score as a covariate of interest, controlling for task order (HE task first vs. LE task first) and task version (index finger = fearful response vs. index finger = neutral response).
Because the AlphaSim correction required a large cluster-level threshold in the whole-brain analysis (over 350 voxels per cluster), an amygdala mask was applied to all analysis 1 and 3 contrasts to prevent false negative results in this small structure. For analysis 2, we hypothesized increased sustained activity in the HE task in either the rACC and/or the DLPFC. Consequently both a DLPFC mask and a rACC mask were applied to the relevant contrasts (HE task-rest and (HE task-rest) – (LE task-rest)). All masks were created using the WFU Pickatlas (Maldjian, Laurienti, Kraft, & Burdette, 2003). The amgydala mask included both right and left amygdala. The DLPFC mask included BA 9 and BA 46. The rACC mask was created from the TD region labeled anterior cingulate, which is centered around the genu of corpus callosum. For all three of these masks, (as in the whole-brain analysis) results were originally thresholded at p<.005, and then corrected for multiple comparisons (p<.05, FWE) using a cluster extent threshold determined using AlphaSim and adjusted for the smaller volume encompassed by the mask.
Behavioral Analysis
Behavioral analyses were performed on RT and accuracy data using SPSS version 18.0 (SPSS, Inc., Chicago, IL; www.spss.com). When correlating behavioral measures with trait anxiety, partial correlations were conducted, controlling for task order (HE task first vs. LE task first) and task version (index finger = fearful response vs. index finger = neutral response). Significance was defined as p < .05, one tailed for the correlations due to our a priori prediction that subjects high in trait anxiety would show more interference and less behavioral adjustment on the HE task compared to the LE task.
Subjects performed a high expectancy (HE) incongruent facial Stroop task.
Subjects performed a low expectancy (LE) incongruent facial Stroop task.
The LE task activates an extensive control network and amygdala on a trial-by-trial basis.
The HE task shows sustained control in right DLPFC and reduced interference.
Subjects higher in anxiety show behavioral and neural impairment in the HE task.
Acknowledgments
Funding was provided by grant RO1 MH059883 awarded to C.S.C. We thank Dennis Thompson and Paul Deramo for assistance with data analysis and Samuel Crowley for assistance with data collection and data analysis.
Footnotes
When the data point representing the lowest RT adjustment (a possible outlier) was removed, r =−.266, p = .0505.
When the data point representing the lowest accuracy (a possible outlier) was removed, r = −.275, p = .045. Please note that data points removed in the accuracy and RT correlations were not from the same individual.
Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at tott0006@tc.umn.edu for more information concerning the stimulus set.
Conflict of interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badre D, Wagner AD. Frontal lobe mechanisms that resolve proactive interference. Cereb Cortex. 2005;15(12):2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Bar Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IMH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7(2):184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. Oxford: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Broadbent D, Broadbent M. Anxiety and attentional bias: State and trait. Cognition and Emotion. 1988;2(3):165–183. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97(4):1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. Neuroimage. 1995;2(4):264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proc Natl Acad Sci U S A. 1999;96(13):7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple-Alford EC, Budayer B. Examination of some aspects of the Stroop Color-Word Test. Percept Mot Skills. 1966;23(3):1211–1214. doi: 10.2466/pms.1966.23.3f.1211. [DOI] [PubMed] [Google Scholar]
- De Pisapia N, Braver TS. A model of dual control mechanisms through anterior cingulate and prefrontal cortex interactions. Neurocomputing. 2006;69:1322–1326. [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler T, Meriau K, Heekeren HR, van der Meer E. Emotional Stroop task: effect of word arousal and subject anxiety on emotional interference. Psychol Res. 2009;73(3):364–371. doi: 10.1007/s00426-008-0154-6. [DOI] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18(6):1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task relevant information. Nat Neurosci. 2005;8(12):1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44(6):1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167(5):545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Gray JR, et al. Anxiety and cognitive efficiency: differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci. 2008;8(3):239–253. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- Funes MJ, Lupianez J, Humphreys G. Sustained vs. transient cognitive control: evidence of a behavioral dissociation. Cognition. 2010;114(3):338–347. doi: 10.1016/j.cognition.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. Optimizing the use of information: strategic control of activation of responses. J Exp Psychol Gen. 1992;121(4):480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS. Personality predicts working-memory-related activation in the caudal anterior cingulate cortex. Cogn Affect Behav Neurosci. 2002;2(1):64–75. doi: 10.3758/cabn.2.1.64. [DOI] [PubMed] [Google Scholar]
- Gray JR, Burgess GC, Schaefer A, Yarkoni T, Larsen RJ, Braver TS. Affective personality differences in neural processing efficiency confirmed using fMRI. Cogn Affect Behav Neurosci. 2005;5(2):182–190. doi: 10.3758/cabn.5.2.182. [DOI] [PubMed] [Google Scholar]
- Hamilton AC, Martin RC. Dissociations among tasks involving inhibition: a single-case study. Cogn Affect Behav Neurosci. 2005;5(1):1–13. doi: 10.3758/cabn.5.1.1. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139(1):181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci U S A. 1998;95(14):8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Kotz SA. Emotion speeds up conflict resolution: a new role for the ventral anterior cingulate cortex? Cereb Cortex. 2011a;21(4):911–919. doi: 10.1093/cercor/bhq157. [DOI] [PubMed] [Google Scholar]
- Kanske P, Kotz SA. Emotion triggers executive attention: anterior cingulate cortex and amygdala responses to emotional words in a conflict task. Hum Brain Mapp. 2011b;32(2):198–208. doi: 10.1002/hbm.21012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Klapp ST. Nonconscious control mimics a purposeful strategy: strength of Stroop-like interference is automatically modulated by proportion of compatible trials. J Exp Psychol Hum Percept Perform. 2007;33(6):1366–1376. doi: 10.1037/0096-1523.33.6.1366. [DOI] [PubMed] [Google Scholar]
- Koster EH, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behav Res Ther. 2004;42(10):1183–1192. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Egner T, Woldorff MG. The neural underpinnings of how reward associations can both guide and misguide attention. J Neurosci. 2011;31(26):9752–9759. doi: 10.1523/JNEUROSCI.0732-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug MK, Carter CS. Adding fear to conflict: a general purpose cognitive control network is modulated by trait anxiety. Cogn Affect Behav Neurosci. 2010;10(3):357–371. doi: 10.3758/CABN.10.3.357. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Silk JS, Dahl RE, Ostapenko L, Kronhaus DM, Phillips ML. Fearful faces influence attentional control processes in anxious youth and adults. Emotion. 2009;9(6):855–864. doi: 10.1037/a0017747. [DOI] [PubMed] [Google Scholar]
- Levens SM, Phelps EA. Emotion processing effects on interference resolution in working memory. Emotion. 2008;8(2):267–280. doi: 10.1037/1528-3542.8.2.267. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Jacoby LL. Stroop process dissociations: the relationship between facilitation and interference. J Exp Psychol Hum Percept Perform. 1994;20(2):219–234. doi: 10.1037//0096-1523.20.2.219. [DOI] [PubMed] [Google Scholar]
- Logan GD, Zbrodoff NJ. When it helps to be misled: Facilitative effects of increasing the frequency of conflict stimuli in a stroop like task. Memory and Cognition. 1979;7:166–174. [Google Scholar]
- MacLeod C, Rutherford EM. Anxiety and the selective processing of emotional information: mediating roles of awareness, trait and state variables, and personal relevance of stimulus materials. Behav Res Ther. 1992;30(5):479–491. doi: 10.1016/0005-7967(92)90032-c. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109(2):163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mathews A, Yiend J, Lawrence AD. Individual differences in the modulation of fear-related brain activation by attentional control. J Cogn Neurosci. 2004;16(10):1683–1694. doi: 10.1162/0898929042947810. [DOI] [PubMed] [Google Scholar]
- Maxwell JS, Shackman AJ, Davidson RJ. Unattended facial expressions asymmetrically bias the concurrent processing of nonemotional information. J Cogn Neurosci. 2005;17(9):1386–1395. doi: 10.1162/0898929054985437. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Padmala S, Bauer A, Pessoa L. Negative emotion impairs conflict-driven executive control. Front Psychol. 2011;2:192. doi: 10.3389/fpsyg.2011.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349(6304):61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990;87(1):256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Salemink E, van den Hout MA, Kindt M. Selective attention and threat: quick orienting versus slow disengagement and two versions of the dot probe task. Behav Res Ther. 2007;45(3):607–615. doi: 10.1016/j.brat.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage. 2006;31(1):468–475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):932–942. [Google Scholar]
- Sichel JL, Chandler KA. The color-word interference test: The effects of varied color-word combinations upon verbal response latency. Journal of Psychology. 1969;72:219–231. [Google Scholar]
- Spielberger CD. Manual for the state trait anxiety inventory. Palo Alto, California: Consulting Psychologists Press; 1983. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, et al. Effects of frontal lobe damage on interference effects in working memory. Cogn Affect Behav Neurosci. 2002;2(2):109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Tzelgov J, Henik A, Berger J. Controlling Stroop effects by manipulating expectations for color words. Mem Cognit. 1992;20(6):727–735. doi: 10.3758/bf03202722. [DOI] [PubMed] [Google Scholar]
- Verkuil B, Brosschot JF, Putman P, Thayer JF. Interacting effects of worry and anxiety on attentional disengagement from threat. Behav Res Ther. 2009;47(2):146–152. doi: 10.1016/j.brat.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, et al. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19(4):1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Wager TD, Feldman Barrett L. From affect to control: Functional specializatio of the insula in motivation and regulation [Electronic Version] 2004 Retrieved from http://www.affective-science.org/pubs/2004/WagerEdfestsubmittedcopy.pdf.
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27(2):323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44(12):1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]