Figure 7.
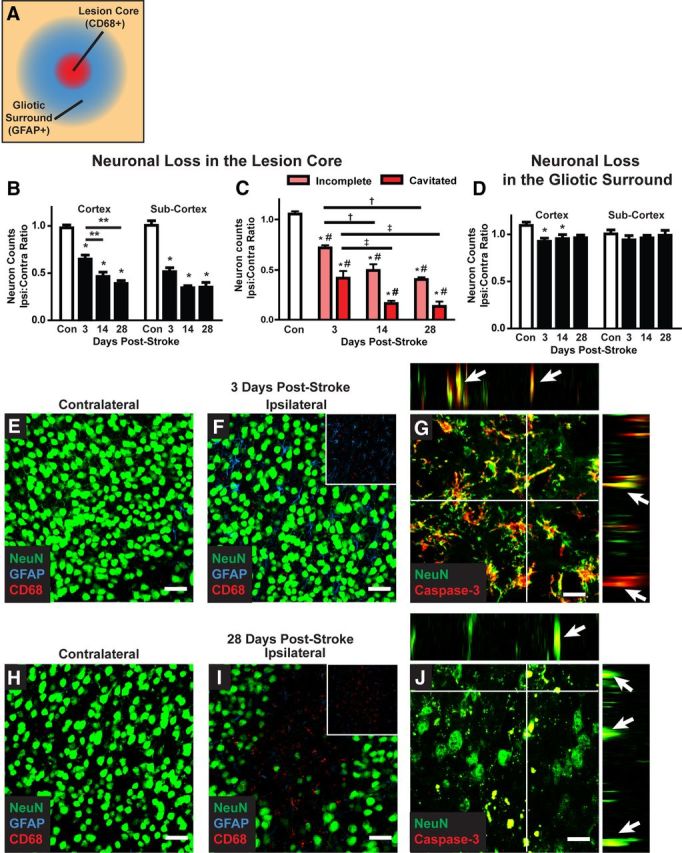
Delayed neuronal cell death after diffuse ischemic stroke. A, Neuronal cell death was evaluated by NeuN immunolabeling. Confocal images (40×; 211 × 211 μm) were generated centered upon CD68-positive cores of diffuse ischemic lesions and in areas 200 μm away that are characterized by an extended field of GFAP-positive reactive astrogliosis. All neurons within the image frame were counted. B, Within the CD68-positive lesion, neuronal cell death in the cortex and subcortical regions was significantly reduced 3 d after stroke (*p < 0.05; **p < 0.01 between time points; ANOVA). In the cortex, neuronal loss was significantly greater at 14 and 28 d after diffuse injury than at 3 d (*p < 0.05, ANOVA). C, When neuronal loss was evaluated based upon lesion type (incomplete vs cavitated), more rapid and complete neuronal loss was observed in cavitated lesions (*p < 0.05, lesion vs control; #p < 0.05, incomplete vs cavitated; †p < 0.05, vs 3 d incomplete; ‡p < 0.05, vs 3 d cavitated; ANOVA). D, In surrounding areas of reactive astrogliosis, a small but significant reduction cortical neuronal counts was observed (*p < 0.05, ANOVA). No difference in subcortical neuronal counts was observed. E, F, H, I, Representative confocal micrographs from CD68- and GFAP-positive lesions and mirror-image contralateral control regions 3 and 28 d after stoke. G, J, Representative NeuN/caspase-3 labeling at 3 and 28 d after injury shows ongoing neuronal cell death up to 28 d after injury. Orthogonal views from z stacks (x–z and y–z) demonstrate colocalization between NeuN and caspase-3 immunoreactivity. Scale bars: E, F, H, I, 25 μm; G, J, 12.5 μm.
