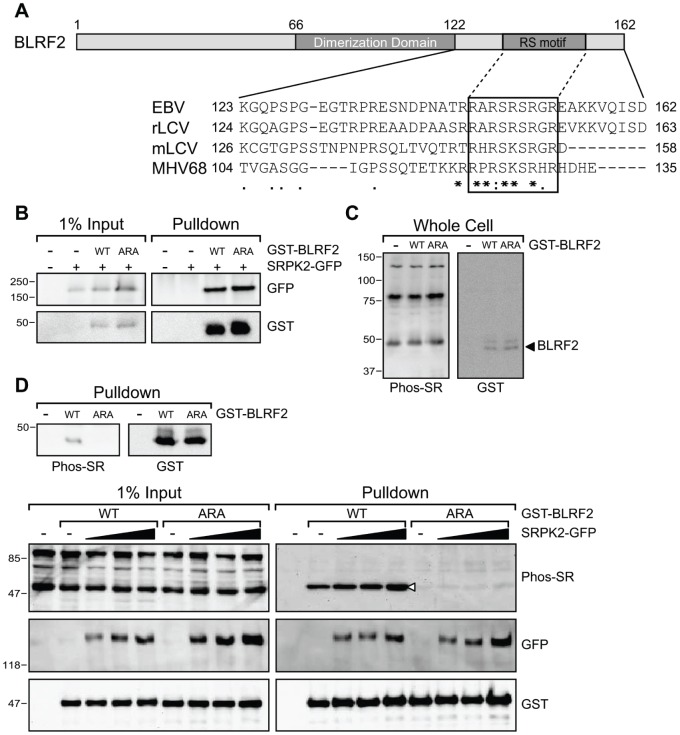
Figure 4. SRPK2 phosphorylates BLRF2.
(A) Schematic of BLRF2 indicating the central dimerization domain (aa 66–122) and the C-terminal RS motif. ClustalW alignment of the C-termini of BLRF2 (aa 123 to 162) and its homologs from the rhesus and marmoset lymphocryptoviruses and murine gammaherpesvirus 68. Degree of conservation is shown at the bottom (* - identical, : - high,. - moderate). A conserved basic domain containing the putative RS motif is highlighted by the box. (B) GST pull-down of 293T cells transfected with GST-BLRF2 wild-type (WT) or GST-BLRF2 SRS-ARA mutant (ARA) in the presence or absence of GFP-SRPK2. Western blot analysis using anti-GFP (top panels) and anti-GST (lower panels) antibodies. Input lysates (1%) are shown in the left panels. (C) Western blots of transfected 293T whole cell lysates probed with anti-Phospho-SR antibody (left) and anti-GST antibody (right). (D) Western blots of GST pull-downs from 293T cells transfected with GST-BLRF2 wild-type or ARA mutant. Phosphorylated GST-BLRF2 is shown in the left panel with anti-Phospho-SR antibody and total GST-BLRF2 level is shown by anti-GST antibody (right panel). (E) Western blot of GST pull-downs of 293T cells transfected with GST-BLRF2 wild-type and ARA mutant along with increasing amounts of SRPK2-GFP. Phosphorylated protein is detected by anti-Phosho-SR antibody (top panels), anti-GFP antibody showed SRPK2-GFP (middle panels) and anti-GST antibody showed total BLRF2 (bottom panels). Input lysates (1%) are shown in the left panels.