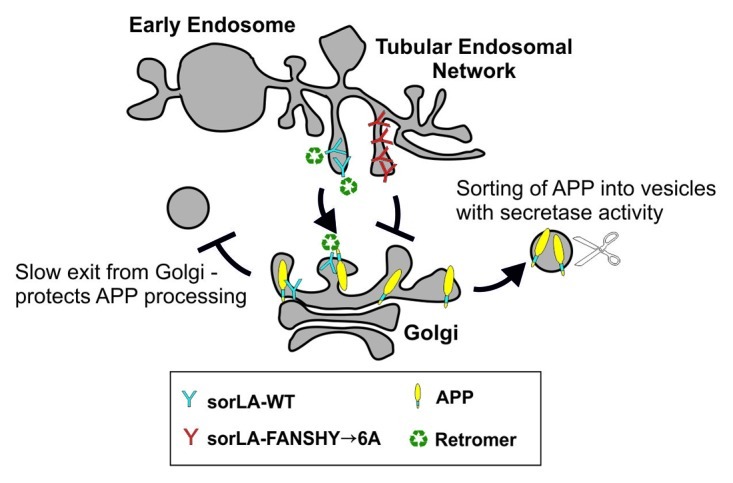
Figure 2. Schematic model of sorLA- and retromer-dependent transport of APP. After mutation of the FANSHY sequence, sorLA is no longer able to interact with retromer. By disrupting this interaction, sorLA cannot keep APP in the Golgi/TGN compartment. APP will then localize into other cellular compartments where APP is more prone to secretase cleavage. Since endosomal processing leads to production of Aβ, increasing endosomal delivery of APP increases the amyloidogenic processing. This change in localization of APP is important because sorLA can only protect against APP processing when located in the TGN. This is supported by the fact that sorLA-FANSHY→6A cannot protect against APP cleavage. When the retromer binding site is deleted sorLA is not recycled back to TGN, but rather stays in the late endosomal compartment, where sorLA has no impact on APP.
