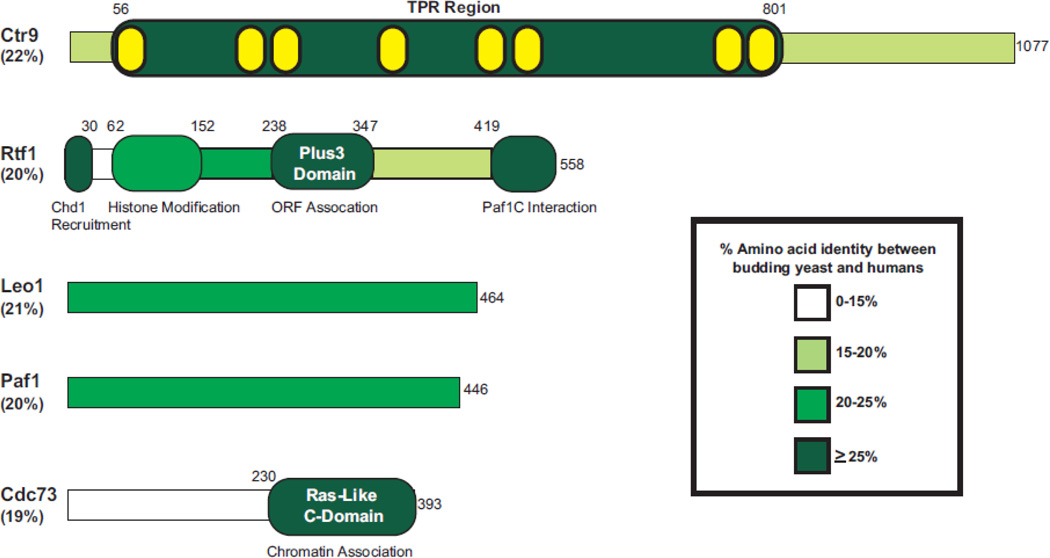
Figure 1. Conservation of the yeast Paf1C subunits.
The five proteins that comprise the Paf1C in budding yeast are depicted. The overall percent amino acid identity between S. cerevisiae and H. sapiens is listed under each protein name. The amino acid identity was determined using a global pairwise alignment algorithm within EMBOSS [177]. Using published literature, regions with defined structures (Rtf1 Plus3 and Cdc73 C-domain) are indicated within each protein and areas of assigned functions are listed below. The predicted TPR motifs found within Ctr9 (depicted internally in yellow) were defined by the SMART domain server [178]. The percent amino acid identity within defined functional and/or structural domains is depicted by color (see legend). Information on domains of the human Paf1C components have been described previously [168].