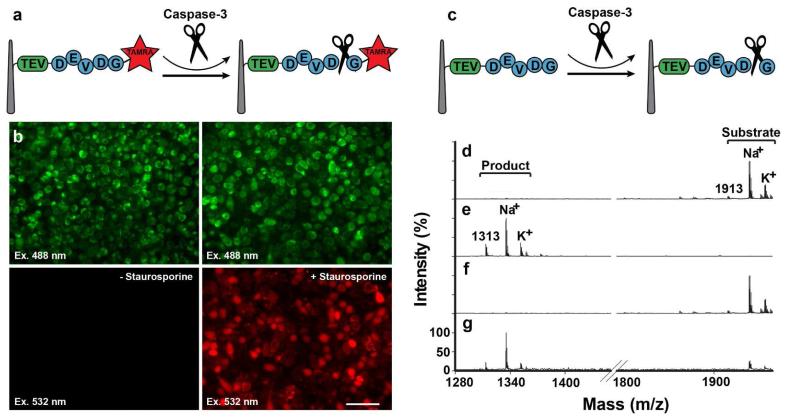
Figure 2.
Assaying caspase-3 activity using a NW-cell sandwich assembly. (a-b) fluorescence-based caspase-3 assay. (a) Assay schematic. (b) TAMRA-labeled substrate peptides were covalently attached to the probe NWs and sandwiched onto HeLa cells immobilized on the NW cell holder. This assembly was then incubated either without (left) or with staurosporine (right), followed by examination for peptide release into the cell cytoplasm by fluorescence microscopy (top panels: all cells visualized by using autofluorescence due to laser excitation at 488 nm; bottom panels: TAMRA-peptide fragments visualized by excitation at 532 nm). (c-g) A mass spectrometry-based caspase-3 assay. (c) Assay schematic. Caspase-3 substrate peptide molecules were covalently attached to the probe NWs, and treated (d) without or (e) with purified caspase 3. Bound peptides were subsequently released with TEV and analyzed by MALDI-TOF (Voyager analyzer). Substrate-containing probe NWs were sandwich-assembled with HeLa cells on the NW cell holder, which was incubated either (f) without or (g) with staurosporine, and processed for TEV cleavage and MALDI-TOF analysis.