Abstract
Objectives
Regular exercise is associated with important benefits in patients with fibromyalgia (FM). Unfortunately, long-term maintenance of exercise after a structured program is rare. The present study tested the efficacy of Motivational Interviewing (MI) to promote exercise and improve symptoms in patients with FM.
Methods
216 patients with FM were randomized to 6 MI sessions (n=107) or an equal number of FM self-management lessons (education control/EC, n=109). Co-primary endpoints were an increase of 30 minutes in moderate-vigorous physical activity and improvement in the Fibromyalgia Impact Questionnaire-Physical Impairment (FIQ-PI) score, assessed at pre-treatment, post-treatment, and 3- and 6-month follow-up. Secondary outcomes included clinically meaningful improvements in FIQ score, pain severity ratings, and a 6-minute walk test.
Results
There were no significant treatment group differences in either co-primary endpoint at 6-month follow-up. However, more MI participants than controls exhibited meaningful improvements in FIQ score at 6-month follow-up (62.9% vs. 49.5%, p=0.06). Compared to EC subjects, MI subjects also displayed a larger increment in their 6-minute walk test (43.9 vs. 24.8 meters, p=0.03). Additionally, MI was superior to EC in increasing the number of hours of physical activity immediately post-intervention and in reducing pain severity both immediately after the intervention and at 3-month follow-up.
Conclusions
Despite a lack of benefits on long term outcome, MI appears to have short-term benefits with respect to self-report physical activity and clinical outcomes. This is the first study in FM that explicitly addresses exercise maintenance as a primary aim.
Keywords: Fibromyalgia, Exercise, Physical activity, Motivational interviewing, Physical function, Pain
INTRODUCTION
Fibromyalgia (FM) affects at least 2% of the general population(1). The individual and societal burden of FM is enormous(2;3). Although drug treatments are available, a physically active lifestyle remains an important component in the management of FM(4). Regular participation in a supervised exercise program is associated with improved symptoms, physical function and global well-being(4). Unfortunately, a lack of long-term exercise maintenance following structured supervised programs is often associated with deteriorating FM symptoms (5;6). FM sufferers who consistently exercise are most likely to maintain initial benefits (5;7); therefore, novel approaches for motivating FM patients to continue regular exercise are warranted.
Motivational Interviewing (MI) is an approach to clinician-client interactions that focuses on enhancing client’s motivation to change(8). An MI clinician helps a client discuss pros and cons of the target behavior (e.g., inactivity); consequently, resolving the client’s ambivalence about the behavior. In this regard, motivation is viewed as something which is elicited in the context of a clinical relationship and interaction, rather than as something that exists only within or imposed on the patient. Numerous studies have reported success with MI to elicit positive health behavior changes (e.g., increasing physical activity) (9–12). Although MI has been used to promote positive exercise behavior among diabetics(13), the use of MI has never been formally tested in the chronic musculoskeletal pain conditions.
The current paper describes the primary results of the Research to Encourage Exercise for Fibromyalgia (REEF) study, which was a randomized attention-controlled trial comparing 6 sessions of telephone-delivered MI to encourage the adoption and/or maintenance of exercise vs. an equal number of FM-relevant educational sessions (education control/EC) over a 12-week period. The co-primary endpoints selected a priori were (1) the proportion of subjects who reported an increase of ≥ 30 minutes/week in moderate-vigorous physical activity (MVPA) measured by Community Health Activities Model Program for Seniors (CHAMPS) and (2) improvement in the Fibromyalgia Impact Questionnaire-Physical Impairment (FIQ-physical impairment) score. We hypothesized that patients receiving MI would report greater pre-treatment to 6-month benefits in the co-primary outcome measures than patients who received the educational control intervention.
MATERIALS AND METHODS
Study Design
Study participants were randomized to either the MI intervention group or the EC group. The MI group received six telephone-delivered exercise-based MI sessions over a 12-week period. The EC group received an equal number of telephone contacts to control for time and therapist attention. Outcome assessments were conducted at baseline, immediate post-treatment, 3-month follow-up and 6-month follow-up. The study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Study procedures, including written informed consent, were approved by Indiana University-Purdue University Indianapolis Institutional Review Board.
Eligibility
All potential participants were referred from specialty or primary care clinics and met the following entry criteria: (a) American College of Rheumatology (ACR) classification criteria for FM(14); (b) average Brief Pain Inventory (BPI) pain severity score ≥ 4; (c) FIQ-physical impairment score ≥ 2; (d) on stable doses of medications for FM ≥ 4 weeks; (d) between 18–65 years old.
Excluded were individuals with (a) known cardiovascular disease; (b) moderate-severe chronic lung disease; (c) uncontrolled hypertension; (d) orthopedic or musculoskeletal conditions that would prohibit moderate-intensity exercise; (e) active suicidal ideation; (f) planned elective surgery during the study period; (g) ongoing unresolved disability claims; (h) inflammatory rheumatic conditions (e.g., rheumatoid arthritis); (i) current use of heart rate lowering medications (e.g., beta-blocker); (j) pregnancy; (k) psychosis; and (l) current participation in MVPA for ≥3 days/week.
Randomization
Subjects were randomized to one of the two treatment arms, with randomization stratified by presence of depression, gender, and referral source (specialty vs. primary care). Allocation to treatment arm was carried out by a computer-generated randomization list with permuted block size of 2.
Supervised exercise training
Both MI and EC participants received an aerobic exercise prescription and two individualized supervised exercise sessions from a qualified fitness instructor who was blinded to treatment assignment. The same fitness instructor, with a bachelor degree in physical education, provided the exercise instructions during the two supervised sessions. The written exercise prescription included the initial exercise intensity (40–50% of the heart rate reserve/HRR), duration (10–12 minutes/session) and frequency (2–3 days/week). Subjects were instructed to gradually increase their total volume of exercise to a maximum of 55–65% of HRR, 28–30 minutes/session, and 3–4 days/week over the ensuing 36 weeks. Details of the exercise prescription have been previously described(15).
After the two supervised exercise sessions, MI subjects received the phone-delivered exercise based MI and the EC group received the phone-delivered education on FM-relevant topics. Each subject had the same interventionist (MI-trained health practitioner or health educator) throughout the study.
Interventions
Exercise-based MI
MI participants had 6 telephone calls over a 12-week period (Supplementary material). The MI-trained health practitioners used an MI handbook(16). The first two MI sessions focused on enhancing patient motivation to exercise by: (a) eliciting self-motivational statements related to: (a) problem recognition and concern about the status quo; (b) intent to participate in graded aerobic exercise; and (c) optimism that exercise-related change is possible. Calls 3 and 4 were devoted to strategies that strengthen commitment to exercise by helping the patient develop a plan for change and reviewing the positive consequences of graded aerobic exercise. The last 2 calls focused on follow-through strategies to prevent relapse of inactivity. Procedures to assure MI fidelity have previously been described(15). Twenty five percent of the audio recorded phone contacts were formally evaluated for treatment integrity using the Motivational Interviewing Treatment Integrity (MITI) method(17). The MITI evaluators were blind to randomization. The MITI included one measure in global rating, and 7 measures in competency ratings in MI-relevant parameters including overall MI adherence. A high global rating score indicates that a therapist is competent in providing MI therapy.
Education control (EC)
Subjects in EC group received didactic health information on the following topics: (a) overview of FM; (b) pain; (c) fatigue; (d) sleep; (e) stress; and (f) living well with FM (Supplementary material).
Procedures used to ensure treatment fidelity in the EC condition have been previously reported(15).
Measures
Co-primary outcome measures
CHAMPS is a 15-minute survey that asks about the frequency and duration of physical activity (PA) in a typical week of the past month. The CHAMPS questionnaire provides a list of various activities ranging from light to vigorous intensity. Research supports the validity, reliability and sensitivity to change of CHAMPS among older adults (18;19). The questionnaire provides measures of estimated hours per week (hrs/week) spent performing light and MVPA (19).
The Fibromyalgia Impact Questionnaire-Physical Impairment (FIQ-physical impairment) is a subscale of the FIQ that assesses a respondent’s ability to engage in 11 different types of physical activity. Scores can range from 0 to 9.99 and a higher score indicates a greater degree of physical impairment. The reliability and validity of the FIQ-physical impairment scale are well established (20;21). Although the treatment intervention was meant to increase exercise behavior, we included FIQ-physical impairment as one of the co-primary outcome measures because of the consistent beneficial effect of exercise on FM-related physical impairment(22). Secondary measures
The FIQ is a disease-specific measure assessing a number of functioning domains related to FM(23). The FIQ includes the FIQ-physical impairment scale (described above), 6 visual analog scales for measuring FM-related symptoms (e.g., pain and fatigue), and two single-item questions assessing work status and overall well-being. A higher score on FIQ (range 0–100) indicates greater severity of global FM symptoms. A 14% or more reduction in the FIQ score is considered a clinically meaningful improvement in the measure (24).
The Brief Pain Inventory (BPI) is a measure of pain with proven reliability and validity across different pain conditions (25;26). BPI pain severity is the average of 4 items asking about current pain and worse, least, and average pain in the past week.
The GT1M ActiGraph (accelerometry) was used as an objective measure of physical activity in a given 7 day period. Research supports the reliability and validity of the accelerometry (27–29). Approximately one week prior to the each assessment visit, participants were instructed to wear the accelerometry around their waist for a minimum of 4 days in a week and for at least 10 waking hours each day. To compute the average number of minutes of MVPA per week we used the following formula: (average # of MVPA per day during weekdays × 5) + (average # of MVPA per day during weekends × 2).
The 6-MWT is an aerobic endurance/fitness measure that is sensitive to change, and significantly related to peak oxygen consumption and the FIQ score(20;30). For this test, subjects walked as far they could in six minutes on a preselected course, with the distance (meters) walked recorded. An increase of at least 40 meters in the 6 minute walk test correlated with improvement in the FIQ score(20;21). The 6-MWT was measured at all assessment time points except for the 3-month follow-up.
Other variables
The Patient Health Questionnaire 8-item Depression Scale (PHQ-8) is a brief self-administered scale which assesses major depressive disorder core symptoms. Scores can range from 0 to 24 and a higher score indicates a greater severity of depressive symptoms(31;32).
We also asked participants at each follow-up time point about any new medications (related to pain or FM); and whether the subject has visited a physician specialist (i.e., neurologist, orthopedist, rheumatologist, pain specialist, physiatrist, or psychiatrist) or allied health practitioner (e.g., nurse practitioner, physical therapist, chiropractor, counselor, etc.) for any pain-related issues.
Statistical analyses
With 97 subjects per group, 94% power was projected to detect a 25% absolute difference (40% vs. 15%) in response rates (i.e., an increase of ≥ 30 minutes in MVPA from baseline as measured by CHAMPS). A treatment response of an increase of ≥ 30 minutes in MVPA from baseline was chosen based on a previous study that showed a significant relationship between maintaining immediate post-program gains in the 6-MWT and ongoing aerobic exercise of ≥ 30 minutes per week at follow-up (33). The resulting sample size also provided 95% power to detect a minimum difference of 1.2 (effect size=0.57) in the improvement of FIQ-physical impairment. To adjust for analyses of 2 primary endpoints, Bonferroni method was used with overall type 1 error rate of 5% (34). In other words, type 1 error rate of 2.5% was applied for each of the two primary endpoints.
Intent to treat analyses was planned for all outcomes. The last observation carried forward approach with at least one follow-up measurement was used for imputing missing values. Fourteen (6.5%) subjects (7 subjects from each group) had none of the follow-up measurements and thus were not included in the analyses. A mixed-effect model was used for testing for mean changes in the outcome variables. All dichotomous outcome variables were tested using repeated measures models and generalized estimating equations (GEEs). The groups were compared for the entire study duration as well as at each specific follow up time point. Proc Genmod (SAS v9.2, SAS Institute, Cary, NC) was used for these analyses. All analyses controlled for the baseline value of the outcome variable and BMI. All analyses met model assumptions including normality for continuous response variables.
RESULTS
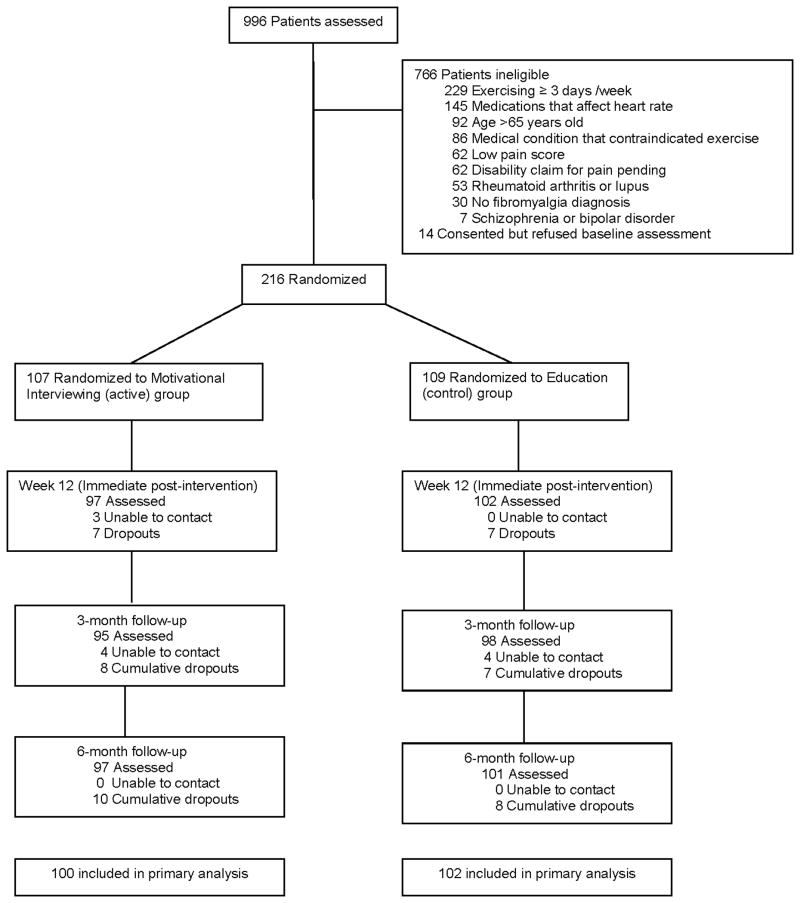
A total of 996 patients were screened for potential participation in the study. Of these, 216 (22%) met the inclusion criteria, enrolled and were randomized to MI (n=107) and EC (n=109). The majority of screening failures were: (a) exercising for ≥3 days a week (30%); (b) use of heart rate lowering medications (19%); (c) aged ≥ 65 years (12%); and (d) presence of medical condition that contraindicated exercise participation (11%) [Figure 1]. Baseline characteristics were similar across treatment groups (p>0.2) except for Body Mass Index (Table 1). Compared to EC subjects, subjects in the MI group were slightly heavier (p=0.07).
Figure 1.
Flow of Participants in the Trial
Table 1.
Baseline characteristics of 216 subjects enrolled in the REEF study*
| Motivational interviewing (n=107) | Education control (n=109) | |
|---|---|---|
| Demographics | ||
| Age in years | 46.0 (11.4) | 45.7 (11.0) |
| Gender, % female | 96 | 95 |
| Ethnicity, % non-Hispanic | 99.1 | 98.2 |
| Race, % white | 90.7 | 86.2 |
| Education, % > high school | 76.6 | 78.9 |
| Marital status, % married | 57.9 | 64.2 |
| Employment, % employed | 57.9 | 49.5 |
| Clinical Variables | ||
| Body mass index (kg/m2) | 32.3 (7.6) | 30.5 (6.6) |
| Duration of fibromyalgia diagnosis (years) | 8.9 (6.4) | 9.1 (7.6) |
| PHQ-8 depression (range 0–24)† | 12.4 (4.8) | 12.7 (5.1) |
| BPI pain severity (range 0–10)† | 5.9 (1.2) | 6.0 (1.4) |
| FIQ-physical impairment (range 0–10)† | 5.3 (1.5) | 5.4 (1.7) |
| FIQ (range 0–100)† | 67.5 (12.0) | 66.6 (13.5) |
| Medications, % prescribed | ||
| Non-tricyclic antidepressants | 66.4 | 57.8 |
| Anticonvulsants | 31.8 | 27.5 |
| Opioid analgesics | 32.7 | 33.0 |
| Physical Activity Measures | ||
| CHAMPS | ||
| MVPA (hrs/wk) | 1.6 (2.7) | 1.9 (4.5) |
| Light only (hrs/wk) | 5.0 (4.5) | 5.0 (4.6) |
| Activity monitor (ActiGraph) | ||
| MVPA (minutes/wk) | 119.3 (88.2) | 118 (98.6) |
| 6-minute walk test (meters) | 483.0 (77.9) | 486.5 (90.7) |
Values are the means (standard deviation) unless otherwise indicated.
Higher score indicates a worse state of health.
Abbreviations: PHQ-8 = Patient Health Questionnaire-8; BPI = Brief Pain Inventory; FIQ = Fibromyalgia Impact Questionnaire; CHAMPS = Community Health Activities Model Program for Seniors; MVPA= moderate-vigorous physical activity. n = number of patients
Physical Activity Outcomes
At the end of the 6-month follow-up, there were no significant differences in the percent of MI and EC participants who reported an increase from baseline of ≥ 30 minutes per week in MVPA (Table 2). Nevertheless, the overall magnitude of improvement in MVPA was significantly greater in the MI than in the EC group [2.2 (0.2) hr/week vs. 1.3 (0.2) hr/week, p=0.01] resulting in a mean group difference of 0.8 (0.3) hr/week. The beneficial effect of MI was most evident immediately after treatment, with a mean group difference of 1.5 (0.6) hr/week (p<0.01). Interestingly, in terms of the accelerometry-based PA, there were no group differences at any time point during the study. However, the 6-MWT favored MI over EC at the end of the study. MI subjects walked 43.9 (6.3) meters farther from their baseline capacity compared to 24.8 (6.3) meters for the EC group (p=0.03).
Table 2.
Treatment Comparisons on Physical Activity Measures
| MI Intervention Group (n=107) | p- value* | Education Control (n=109) | p- value* | P-value (MI vs. EC) | |
|---|---|---|---|---|---|
| PRIMARY OUTCOME | |||||
| % subjects with ≥ 30-minute increment of MVPA (CHAMPS) at 6-mo follow-up¥ | 54 (54%) | 53 (52%) | 0.89 | ||
| SECONDARY OUTCOMES | |||||
| Change in time spent on MVPA (CHAMPS: hours/wk) from baseline† | |||||
| Overall | 2.2 (0.2) | 1.3 (0.2) | 0.01 | ||
| Week 12 | 2.6 (0.4) | <0.01 | 1.1 (0.4) | <0.01 | <0.01 |
| 3-mo follow-up | 1.9 (0.4) | <0.01 | 1.6 (0.4) | <0.01 | 0.67 |
| 6-mo follow-up | 2.0 (0.4) | <0.01 | 1.2 (0.4) | <0.01 | 0.19 |
| Change in time spent on MVPA (ActiGraph: minutes/wk) from baseline | |||||
| Overall | −21.64 (6.8) | −17.74 (6.7) | 0.68 | ||
| Week 12 | −6.26 (9.7) | 0.52 | −27.02 (10.0) | <0.01 | 0.14 |
| 3-mo follow-up | −24.04 (12.6) | 0.06 | −3.57 (12.3) | 0.77 | 0.25 |
| 6-mo follow-up | −34.63 (8.8) | <0.01 | −22.63 (8.9) | <0.05 | 0.34 |
| Six-minute walk test (meters)¶ | |||||
| Overall | 38.5 (4.4) | 21.4 (4.4) | <0.01 | ||
| Week 12 | 33.0 (6.2) | <0.01 | 18.1 (6.0) | <0.01 | 0.09 |
| 6-mo follow-up | 43.9 (6.3) | <0.01 | 24.8 (6.3) | <0.01 | 0.03 |
Used last observation carried forward approach. Data for the secondary outcomes are expressed as mean changes (standard error) from study entry. All analyses controlled for baseline value of the outcome variable and body mass index.
p-values for testing within group mean changes from baseline.
Positive change in CHAMPS represents an increased in the number of hours/week of moderate-vigorous physical activity (MVPA) at follow-up.
Positive change in the six-minute walk test represents an ability to walk longer distance at follow-up. The six-minute walk test was not measured at 3-month follow-up. CHAMPS: Community Health Activities Model Program for Seniors.
Clinical Outcomes
The magnitude of improvement in the FIQ-physical impairment from study entry to 6-month follow-up was not significantly different between groups (Table 3). However, compared to EC, more MI subjects achieved a clinically meaningful improvement in their FIQ score at week 12 (59.8% vs. 46.5%, p=0.07) and at 6-month follow-up (62.9% vs. 49.5%, p=0.06). Further, compared to EC subjects, MI subjects reported greater improvement in BPI pain severity that was evident immediately post-intervention and at 3-month follow-up, although not at 6-month follow-up.
Table 3.
Treatment Comparisons on Clinical (Symptoms) Measures
| MI Intervention Group (n=107) | p-value* | Education Control (n=109) | p-value* | P-value (MI vs. EC) | |
|---|---|---|---|---|---|
| PRIMARY OUTCOME | |||||
| Change in FIQ-physical impairment at 6-mo follow-up† | −1.7 (0.2) | <0.01 | −1.4 (0.2) | <0.01 | 0.39 |
| SECONDARY OUTCOMES | |||||
| At least 14% improvement in FIQ | |||||
| Overall | 82 (82.0%) | 70 (68.6%) | 0.03 | ||
| Week 12 | 58 (59.8%) | 47 (46.5%) | 0.07 | ||
| 3-mo follow-up | 48 (51.6%) | 40 (41.7%) | 0.19 | ||
| 6-mo follow-up | 61 (62.9%) | 50 (49.5%) | 0.06 | ||
| BPI pain severity† | |||||
| Overall | −1.2 (0.10) | −0.9 (0.10) | 0.01 | ||
| Week 12 | −1.2 (0.17) | <0.01 | −0.8 (0.16) | <0.01 | <0.05 |
| 3-mo follow-up | −1.1 (0.18) | <0.01 | −0.6 (0.18) | <0.01 | 0.02 |
| 6-mo follow-up | −1.2 (0.18) | <0.01 | −1.2 (0.18) | <0.01 | 0.90 |
| PHQ-8 depression† | |||||
| Overall | −2.6 (0.3) | −2.7 (0.3) | 0.69 | ||
| Week 12 | −3.3 (0.5) | <0.01 | −2.8 (0.5) | <0.01 | 0.46 |
| 3-mo follow-up | −2.4 (0.5) | <0.01 | −2.6 (0.5) | <0.01 | 0.71 |
| 6-mo follow-up | −2.2 (0.5) | <0.01 | −2.8 (0.5) | <0.01 | 0.34 |
Data for FIQ-physical impairment (used last observation carried forward approach).
p-values for testing within group mean changes from baseline. BPI pain severity and PHQ-8 depression are expressed as mean changes (standard error) of scores from study entry. Negative change in FIQ-physical impairment, BPI pain severity or PHQ-8 depression indicates an improvement in symptoms. FIQ-physical impairment, BPI pain severity and PHQ-8 were adjusted for values at study entry and body mass index. FIQ: Fibromyalgia impact questionnaire; BPI: Brief pain inventory; PHQ-8: Patient health assessment-8
The overall change in depression severity (PHQ-8) was not significantly different between groups. There was no significant group difference in self-report use of new FM-related or pain medications throughout the study (MI vs. EC, 20% vs. 28%, p=0.18). However, a significantly lower proportion of MI than EC subjects (78% vs. 91%, p=0.01) reported visiting a physician specialist or allied health practitioner for any pain-related issue during the entire study period.
Treatment Dose and Fidelity Assessment
The average MI subject completed a mean (SD) of 5.7 (0.5) sessions compared to 5.5 (1.0) for the average EC subject (p=0.13). Similarly, the MI-trained practitioners and the health educator (for the EC) spent about same amount of time [24.0 (10) vs. 24.8 (7) minutes/session, p=0.7)] per session with their respective clients.
Table 4 shows that MI participants received sessions where the MI-trained practitioner showed solid adherence to the principles of MI 80% of time. The health educator (EC group) showed proficient adherence to the principles of MI 20% of the time. On a scale from 5 to 25, the mean (SE) global rating for the MI sessions was 22.02 (2.83) vs. 13.67 (2.79) for the EC educational sessions. These findings show that fidelity to the intervention was generally well maintained, although it was interesting to note that participants in the EC group did get ‘some’ MI interactions (or, at least interactions that were coded as MI treatment).
Table 4.
Treatment Fidelity Assessment
| MI sessions (N=265) | Education control sessions (N=256) | P value | |
|---|---|---|---|
| MITI global rating+ (range 5–25), mean (SD) | 22.02 (2.83) | 13.67 (2.79) | <0.001 |
| MITI competence summary† | |||
| Global spirit | 206 (78%) | 28 (11%) | < 0.001 |
| Global empathy | 225 (86%) | 59 (23%) | < 0.001 |
| Global direction | 193 (73%) | 216 (84%) | 0.002 |
| Reflection/Question ratio | 111 (42%) | 32 (13%) | < 0.001 |
| Percent open question | 104 (40%) | 36 (14%) | < 0.001 |
| Percent complex reflection | 196 (76%) | 75 (30%) | < 0.001 |
| Percent MI adherent | 206 (80%) | 50 (20%) | < 0.001 |
MITI global rating is a 5-item scale that covers empathy, direction, collaboration, evocation and autonomy/support.
Proportion of recorded sessions with solid MI competence rating. N= number of recorded sessions. MITI: Motivational Interviewing Treatment Integrity. P-values are from nested ANOVA (sessions within therapist) for continuous variable or from logistic regression analyses (multinomial/polytomous) for categorical data.
DISCUSSION
In this randomized controlled trial, MI was not superior to an education control intervention in reducing physical impairment or in increasing self-report physical activity from pre-treatment to six months after completion of therapy. However, over the short term, MI resulted in significant improvement in pain severity and self-report physical activity, relative to control condition. Additionally, MI was associated with a clinically significant increased walking distance (i.e., 6-MWT) and a trend towards greater proportion of subjects achieving a clinically significant improvement in FM symptom severity.
Many studies have reported success with MI to elicit positive health-related behavior changes(10;11;35;36), but research investigating the use of MI to promote exercise is relatively new. In the general medical patient population, MI has been shown to have a medium effect size of 0.5 for increasing physical activity(37). MI has also been shown to be effective in increasing exercise among diabetics(13). However, although short-term improvements were observed in the present study, the long term outcome data on the primary outcome variables indicated a lack of efficacy of MI relative to education control. There are a number of possible reasons for this finding. First, unlike other patient populations, many patients with chronic pain are fearful that exercise might exacerbate their existing pain or result in a new injury(6). Fear of pain and injury can be a potent inhibitory factor for participation in regular exercise regimens(38) and may shorten participation time among those asked to engage in physical activity(39). Such fears were not specifically addressed in the MI intervention used in this study. Second, although subjects received an individualized low-moderate intensity aerobic exercise prescription accompanied by two individualized supervised exercise sessions, the minimal amount of direct exercise supervision in the current study may have prevented the study participants from acquiring the necessary skills, including modifying the exercise prescription during exercise-induced pain flares. FM patients who are unable to self-adjust their exercise regimen during pain flares are more likely to quit exercise(40). Thus, although the MI group might have had more motivation to exercise than the control participants post-treatment, they may have lacked some specific skills needed to maintain a home exercise program. A third possible explanation is that the control subjects “received” MI treatment that had been coded by experts 20% of the time. Had the education interventionist been taught to avoid MI strategies, a larger difference between the conditions might have emerged.
In the current study, it is reassuring to note that a 2.5-hours worth of phone-delivered MI was beneficial at least in the short term. In comparison to other psycho-educational intervention studies in other pain conditions (e.g., chronic low back pain and osteoarthritis), MI appears to have distinct effects over and above education. For example, compared to usual care, a six-week to six-month course of arthritis self management program has been shown to improve self-report exercise frequency(41–44). In contrast, cognitive and/or behavioral principle-based interventions were not more effective in boosting exercise maintenance when compared to an attention (or active) control (45–49). Considering that the control group in the current study received an intensive FM-relevant educational intervention, the observed beneficial effects of MI, although short-lived, were likely real.
Although MI was associated with short term improvement in self-report PA and clinical symptoms, improvement in the accelerometry-based PA was not observed at any time points during the study. There are 2 possible explanations. First, overestimation of physical activity levels is common among the general, sedentary population (>60%)(50), and could be even higher in patients with FM, who are typically sedentary and frequently report cognitive deficiencies in attention, concentration and memory(51;52). However, given the RCT design of our study, the overestimation rate in each group should be about the same; and therefore, would not explain the short term beneficial effects of MI on self-report PA. Second, hip-worn accelerometers, like the one used in this study, primarily capture the movement associated with dynamic lower-body activity(53). Common household or lawn and garden activities, which typically require greater upper-body muscular involvement, therefore, may not always be detected(54;55). These types of activities are particularly prevalent among women and often reported as at least moderate intensity(56). For that reason, they are likely contributors to the weekly accumulation of self-report physical activity that may go undetected by accelerometry. As no single quantitative measure of physical activity is superior, combining two or more methods (e.g., wrist-worn and hip-worn accelerometers and self-report PA measure) would allow strengths of one method to help compensate for weaknesses of the other is what experts would recommend(57).
Our study has several limitations. First, we did not collect activity logs (or diaries) to document daily activity levels. Activity logs could help alleviate the potential problem of poor memory. Inclusion of an activity log may have offered additional useful insight for interpreting the results. Second, although the demographic characteristics of the study participants were comparable to other psychological-based clinical trials in FM(58), the generalizability of our results to males or to ethnic minorities would need to be demonstrated. Third, given that CHAMPS measures physical activity (i.e., household chores, sports, recreational activities, and exercise), the use of CHAMPS in our study may be problematic considering that subjects received instructions for exercise (e.g., walk, 3 days per week, 15 minutes per day, 50% of heart rate reserve) and not physical activity. The use of CHAMPS in our inactive FM patients was deemed reasonable because of the well established psychometric properties of CHAMPS in older sedentary adults (18;19); the absence of a validated measure of exercise behavior for FM; and that 15 of the 40 listed physical activities in CHAMPS do relate to exercise (e.g., chair exercises, light strength training such as handheld weights, etc.).
Clearly, MI alone is insufficient to increase physical activity and sustain long-term clinical benefits. Additional interventions may be needed to achieve more robust outcomes. Motivating FM patients to continue regular exercise would likely require a combination of different social (e.g., group exercise program) and behavioral strategies (e.g., providing reminders and feedback through the use of interactive voice response or pedometer) to impact long term clinical outcome(59–61).
CONCLUSIONS
REEF was the first large scale clinical trial of exercise-based MI in patients with chronic musculoskeletal pain. With a 92% study completion rate, REEF was adequately powered to answer the co-primary outcome measures on physical activity and pain-related disability. Moreover, REEF successfully delivered a treatment intervention that was faithful to the principles and spirits of MI. Despite the lack of long-term effects of the MI intervention used here, MI appears to have promising short-term benefits with regards to self-report physical activity and clinical outcomes.
Supplementary Material
Acknowledgments
Funding: National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant number: 1RO1AR054324-01A1). The funding institution did not play any role in the collection, analysis, data interpretation, and writing of the manuscript.
Footnotes
No conflicts of interest to declare
Clinical trial registration: NCT00573612
Reference List
- 1.Wolfe F, Ross K, Anderson J, et al. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 2.Winkelmann A, Perrot S, Schaefer C, et al. Impact of fibromyalgia severity on health economic costs: results from a European cross-sectional study. Appl Health Econ Health Policy. 2011;9:125–36. doi: 10.2165/11535250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Lachaine J, Beauchemin C, Landry PA. Clinical and economic characteristics of patients with fibromyalgia syndrome. Clin J Pain. 2010;26:284–90. doi: 10.1097/AJP.0b013e3181cf599f. [DOI] [PubMed] [Google Scholar]
- 4.Busch AJ, Thille P, Barber KA, et al. Best practice: E-Model--prescribing physical activity and exercise for individuals with fibromyalgia. Physiother Theory Pract. 2008;24:151–66. doi: 10.1080/09593980701686872. [DOI] [PubMed] [Google Scholar]
- 5.Gowans SE, Dehueck A, Voss S, et al. Six-month and one-year followup of 23 weeks of aerobic exercise for individuals with fibromyalgia. Arthritis Rheum. 2004;51:890–8. doi: 10.1002/art.20828. [DOI] [PubMed] [Google Scholar]
- 6.Ramsay C, Moreland J, Ho M, et al. An observer-blinded comparison of supervised and unsupervised aerobic exercise regimens in fibromyalgia. Rheumatology (Oxford) 2000;39:501–5. doi: 10.1093/rheumatology/39.5.501. [DOI] [PubMed] [Google Scholar]
- 7.Lemstra M, Olszynski WP. The effectiveness of multidisciplinary rehabilitation in the treatment of fibromyalgia: a randomized controlled trial. Clin J Pain. 2005;21:166–74. doi: 10.1097/00002508-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York: Guilford Press; 1991. [Google Scholar]
- 9.van Keulen HM, Mesters I, Ausems M, et al. Tailored print communication and telephone motivational interviewing are equally successful in improving multiple lifestyle behaviors in a randomized controlled trial. Ann Behav Med. 2011;41:104–18. doi: 10.1007/s12160-010-9231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris KJ, Catley D, Good GE, et al. Motivational interviewing for smoking cessation in college students: a group randomized controlled trial. Prev Med. 2010;51:387–93. doi: 10.1016/j.ypmed.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walton MA, Chermack ST, Shope JT, et al. Effects of a brief intervention for reducing violence and alcohol misuse among adolescents: a randomized controlled trial. JAMA. 2010;304:527–35. doi: 10.1001/jama.2010.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groeneveld IF, Proper KI, van der Beek AJ, et al. Sustained body weight reduction by an individual-based lifestyle intervention for workers in the construction industry at risk for cardiovascular disease: results of a randomized controlled trial. Prev Med. 2010;51:240–6. doi: 10.1016/j.ypmed.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Lohmann H, Siersma V, Olivarius NF. Fitness consultations in routine care of patients with type 2 diabetes in general practice: an 18-month non-randomised intervention study. BMC Fam Pract. 2010;11:83. doi: 10.1186/1471-2296-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 15.Ang DC, Kaleth AS, Bigatti S, et al. Research to Encourage Exercise for Fibromyalgia (REEF): Use of motivational interviewing design and method. Contemp Clin Trials. 2010 doi: 10.1016/j.cct.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ang D, Kesavalu R, Lydon JR, et al. Exercise-based motivational interviewing for female patients with fibromyalgia: a case series. Clin Rheumatol. 2007 doi: 10.1007/s10067-007-0587-0. [DOI] [PubMed] [Google Scholar]
- 17.Moyers TB, Martin T, Manuel JK, et al. Assessing competence in the use of motivational interviewing. J Subst Abuse Treat. 2005;28:19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Harada ND, Chiu V, King AC, et al. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33:962–70. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Stewart AL, Mills KM, King AC, et al. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Mannerkorpi K, Nyberg B, Ahlmen M, et al. Pool exercise combined with an education program for patients with fibromyalgia syndrome. A prospective, randomized study. J Rheumatol. 2000;27:2473–81. [PubMed] [Google Scholar]
- 21.Mannerkorpi K, Ahlmen M, Ekdahl C. Six- and 24-month follow-up of pool exercise therapy and education for patients with fibromyalgia. Scand J Rheumatol. 2002;31:306–10. doi: 10.1080/030097402760375223. [DOI] [PubMed] [Google Scholar]
- 22.Busch AJ, Schachter CL, Overend TJ, et al. Exercise for fibromyalgia: a systematic review. J Rheumatol. 2008;35:1130–44. [PubMed] [Google Scholar]
- 23.Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23:S154–S162. [PubMed] [Google Scholar]
- 24.Bennett RM, Bushmakin AG, Cappelleri JC, et al. Minimal clinically important difference in the fibromyalgia impact questionnaire. J Rheumatol. 2009;36:1304–11. doi: 10.3899/jrheum.081090. [DOI] [PubMed] [Google Scholar]
- 25.Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50:2974–84. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 26.Tan G, Jensen MP, Thornby JI, et al. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–7. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Welk GJ, Schaben JA, Morrow JR., Jr Reliability of accelerometry-based activity monitors: a generalizability study. Med Sci Sports Exerc. 2004;36:1637–45. [PubMed] [Google Scholar]
- 28.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37:S531–S543. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 29.Kop WJ, Lyden A, Berlin AA, et al. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 30.King S, Wessel J, Bhambhani Y, et al. Validity and reliability of the 6 minute walk in persons with fibromyalgia. J Rheumatol. 1999;26:2233–7. [PubMed] [Google Scholar]
- 31.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345–59. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Gowans SE, Dehueck A, Voss S, et al. A randomized, controlled trial of exercise and education for individuals with fibromyalgia. Arthritis Care Res. 1999;12:120–8. doi: 10.1002/1529-0131(199904)12:2<120::aid-art7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 35.Tappin DM, Lumsden MA, Gilmour WH, et al. Randomised controlled trial of home based motivational interviewing by midwives to help pregnant smokers quit or cut down. BMJ. 2005;331:373–7. doi: 10.1136/bmj.331.7513.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samet JH, Horton NJ, Meli S, et al. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antivir Ther. 2005;10:83–93. doi: 10.1177/135965350501000106. [DOI] [PubMed] [Google Scholar]
- 37.Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71:843–61. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- 38.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–32. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 39.de GM, Peters ML, Vlaeyen JW. Fear of pain, physical performance, and attentional processes in patients with fibromyalgia. Pain. 2003;104:121–30. doi: 10.1016/s0304-3959(02)00487-6. [DOI] [PubMed] [Google Scholar]
- 40.Jones KD, Liptan GL. Exercise interventions in fibromyalgia: clinical applications from the evidence. Rheum Dis Clin North Am. 2009;35:373–91. doi: 10.1016/j.rdc.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Barlow JH, Turner AP, Wright CC. A randomized controlled study of the Arthritis Self-Management Programme in the UK. Health Educ Res. 2000;15:665–80. doi: 10.1093/her/15.6.665. [DOI] [PubMed] [Google Scholar]
- 42.Fries JF, Carey C, McShane DJ. Patient education in arthritis: randomized controlled trial of a mail-delivered program. J Rheumatol. 1997;24:1378–83. [PubMed] [Google Scholar]
- 43.Nour K, Laforest S, Gauvin L, et al. Behavior change following a self-management intervention for housebound older adults with arthritis: an experimental study. Int J Behav Nutr Phys Act. 2006;3:12. doi: 10.1186/1479-5868-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yip YB, Sit JW, Fung KK, et al. Effects of a self-management arthritis programme with an added exercise component for osteoarthritic knee: randomized controlled trial. J Adv Nurs. 2007;59:20–8. doi: 10.1111/j.1365-2648.2007.04292.x. [DOI] [PubMed] [Google Scholar]
- 45.Asenlof P, Denison E, Lindberg P. Individually tailored treatment targeting activity, motor behavior, and cognition reduces pain-related disability: a randomized controlled trial in patients with musculoskeletal pain. J Pain. 2005;6:588–603. doi: 10.1016/j.jpain.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Bernaards CM, Ariens GA, Knol DL, et al. The effectiveness of a work style intervention and a lifestyle physical activity intervention on the recovery from neck and upper limb symptoms in computer workers. Pain. 2007;132:142–53. doi: 10.1016/j.pain.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Jensen IB, Bergstrom G, Ljungquist T, et al. A randomized controlled component analysis of a behavioral medicine rehabilitation program for chronic spinal pain: are the effects dependent on gender? Pain. 2001;91:65–78. doi: 10.1016/s0304-3959(00)00420-6. [DOI] [PubMed] [Google Scholar]
- 48.Smeets RJ, Vlaeyen JW, Hidding A, et al. Active rehabilitation for chronic low back pain: cognitive-behavioral, physical, or both?. First direct post-treatment results from a randomized controlled trial [ISRCTN22714229] BMC Musculoskelet Disord. 2006;7:5. doi: 10.1186/1471-2474-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veenhof C, Koke AJ, Dekker J, et al. Effectiveness of behavioral graded activity in patients with osteoarthritis of the hip and/or knee: A randomized clinical trial. Arthritis Rheum. 2006;55:925–34. doi: 10.1002/art.22341. [DOI] [PubMed] [Google Scholar]
- 50.van Sluijs EM, Griffin SJ, van Poppel MN. A cross-sectional study of awareness of physical activity: associations with personal, behavioral and psychosocial factors. Int J Behav Nutr Phys Act. 2007;4:53. doi: 10.1186/1479-5868-4-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glass JM. Review of cognitive dysfunction in fibromyalgia: a convergence on working memory and attentional control impairments. Rheum Dis Clin North Am. 2009;35:299–311. doi: 10.1016/j.rdc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Leavitt F, Katz RS. Distraction as a key determinant of impaired memory in patients with fibromyalgia. J Rheumatol. 2006;33:127–32. [PubMed] [Google Scholar]
- 53.Westerterp KR. Assessment of physical activity: a critical appraisal. Eur J Appl Physiol. 2009;105:823–8. doi: 10.1007/s00421-009-1000-2. [DOI] [PubMed] [Google Scholar]
- 54.Bassett DR, Jr, Ainsworth BE, Swartz AM, et al. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc. 2000;32:S471–S480. doi: 10.1097/00005768-200009001-00006. [DOI] [PubMed] [Google Scholar]
- 55.Bassett DR., Jr Validity and reliability issues in objective monitoring of physical activity. Res Q Exerc Sport. 2000;71:S30–S36. [PubMed] [Google Scholar]
- 56.Brownson RC, Eyler AA, King AC, et al. Patterns and correlates of physical activity among US women 40 years and older. Am J Public Health. 2000;90:264–70. doi: 10.2105/ajph.90.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Treuth MS. Applying multiple methods to improve the accuracy of activity assessments. In: Welk GJ, editor. Physical activity assessments for health related research. Champaign, Illinois: Human Kinetics Publishers; 2002. [Google Scholar]
- 58.Glombiewski JA, Sawyer AT, Gutermann J, et al. Psychological treatments for fibromyalgia: a meta-analysis. Pain. 2010;151:280–95. doi: 10.1016/j.pain.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010:CD005956. doi: 10.1002/14651858.CD005956.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naylor MR, Keefe FJ, Brigidi B, et al. Therapeutic Interactive Voice Response for chronic pain reduction and relapse prevention. Pain. 2008;134:335–45. doi: 10.1016/j.pain.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.