Abstract
Previous studies have documented the positive effects of mindfulness meditation on executive control. What has been lacking, however, is an understanding of the mechanism underlying this effect. Some theorists have described mindfulness as embodying two facets—present moment awareness and emotional acceptance. Here, we examine how the effect of meditation practice on executive control manifests in the brain, suggesting that emotional acceptance and performance monitoring play important roles. We investigated the effect of meditation practice on executive control and measured the neural correlates of performance monitoring, specifically, the error-related negativity (ERN), a neurophysiological response that occurs within 100 ms of error commission. Meditators and controls completed a Stroop task, during which we recorded ERN amplitudes with electroencephalography. Meditators showed greater executive control (i.e. fewer errors), a higher ERN and more emotional acceptance than controls. Finally, mediation pathway models further revealed that meditation practice relates to greater executive control and that this effect can be accounted for by heightened emotional acceptance, and to a lesser extent, increased brain-based performance monitoring.
Keywords: anterior cingulate cortex, emotion, error-related negativity, meditation, mindfulness
INTRODUCTION
Scientific interest in meditation and mindfulness has exploded in the last decade, fueling study after study demonstrating various positive outcomes of mindfulness meditation. When considering the fundamental principles behind mindfulness meditation practice—such as present moment awareness and mindful acceptance of emotional states (Cardaciotto et al., 2008)—it is not surprising that meditation practice has been shown to enhance executive control (e.g. Jha et al., 2007) and improve self-regulation (Brown and Deci, 2003; Chambers et al., 2008). Such findings have contributed greatly to clinical theory and have even been extended to projects involving the US military (Stanley et al., 2011). Given the significance and overall practicality of this topic, it is quite apparent why so much research has explored the links between meditation and control. But, why exactly does meditation practice improve executive control? Even though the relationship between meditation and control is robust, an understanding of the precise mechanisms underlying this effect is lacking. In the current experiment, we attempt to do just this: to uncover how and why meditation is related to enhanced executive control; and we do so by relating meditation practice to brain-based performance monitoring.
MEDITATION AND EXECUTIVE CONTROL
Finding its roots in Buddhist tradition, mindfulness meditation is thought to consist primarily of two facets, present moment awareness and mindful acceptance of feelings and emotional states (Cardaciotto et al., 2008). Although some theorists have suggested that there may exist additional facets (e.g. gratitude, non-striving, ‘lovingkindness’, etc.) (Kabat-Zinn, 1990), most definitions of mindfulness highlight two key constructs: (i) the behavior that is conducted (i.e. acknowledging thoughts and feelings), which can be conceptualized as awareness and (ii) the manner in which this behavior is conducted (i.e. openly accepting and approving of one’s thoughts and feelings), which can be conceptualized as acceptance (Cardaciotto et al., 2008). Practitioners of meditation are taught to attend to all thoughts, sensations and feelings, but also to attend to them non-judgmentally. In other words, it is important to acknowledge all thoughts that enter the mind (attention), but it is also important to avoid getting caught up in the internal stories and emotions associated with them (acceptance; Kabat-Zinn, 1994). As such, it seems that practicing meditation should equip individuals with a set of skills ideal for regulating attention and fostering control.
Executive control entails a number of cognitive processes such as planning, acquiring rules, attending to relevant stimuli and finally initiating appropriate behavior while inhibiting inappropriate behavior. Miyake and colleagues have broken down executive functioning into three key constructs: (i) mental set shifting; (ii) information updating and monitoring; and (iii) the inhibition of pre-potent responses (Miyake et al., 2000). As such, executive control allows people to overcome impulses and override automatic behavior. Also referred to as ‘self-control’, this cornerstone ability is essential for things like intellectual performance (Schmeichel et al., 2003), impression management (Vohs et al., 2005) and even emotion regulation (Compton et al., 2008).
Cognitive neuroscientists have described an important aspect of executive control as a process that compares current behavior to an ideal desired outcome, and this process is supported by the anterior cingulate cortex (ACC), which feeds the outcome of this comparison to the dorsolateral prefrontal cortex (DLPFC) (Kerns et al., 2004). For instance, neuroscientists talk about ‘performance monitoring’, ‘conflict-monitoring’ or ‘error monitoring’ to refer to a process that detects incongruity between the mental representations of intended and actual responses or between the representation of two conflicting response tendencies (Botvinick et al., 2001; Holroyd and Coles, 2002). For example, during a Stroop task—a canonical measure of the inhibition facet of executive control (Miyake et al., 2000)—participants are presented with words and are asked to name the color in which these words are presented. During incongruent trials (i.e. ‘red’ printed in green), there is a high degree of response conflict, which signals the need for deliberate control because the relatively automatic behavior (word reading) must be inhibited in favor of a less automatic behavior (naming the color of the ink). In this task, participants exhibit executive control when they override their automatic impulse.
Such performance monitoring relates directly to meditation because the act of meditation can be conceptualized as a type of performance monitoring itself, requiring practitioners to monitor their minds and return their focus back to the present moment. Deikman (1966) suggested that deautomatization and deliberation are needed to overcome pre-potent responses, and that this can be achieved by the reinvestment of attention in actions. Critically, this is precisely what the act of meditation entails, requiring the practitioner to focus their attention to the present, on a moment-by-moment basis (Marlatt and Kristeller, 1999). For example, if a meditator automatically begins to engage in rumination upon the recognition of a particular thought during practice, executive control is required to halt the process of rumination and bring focus back to the present moment. In other words, meditation requires ‘cognitive flexibility’ (Moore and Malinowski, 2009), making it an ideal training tool for the cultivation of executive control.
Indeed, there exists a wealth of evidence to support the association between meditation practice and improved executive function. Engaging in short-term meditation practice improves executive function, as measured by performance on the Stroop task (Wenk-Sormaz, 2005). Moore and Malinowski (2009) were able to extend this finding by showing that meditators exhibit less Stroop interference than do control participants. Related work conducted by Jha and colleagues (2007) using the Attention Network Test (Fan et al., 2002), has found that experienced meditators excel at conflict monitoring. Tang and colleagues (2007) provided additional evidence for this effect by showing that just 5 days of brief meditation training improved conflict monitoring for this same test. Finally, related work investigating attentional control has demonstrated that participants who completed a 10-day intensive meditation retreat showed significant improvements in attentional switching on the Internal Switching Task (Chambers et al., 2008). Semple (2010) solidified this effect by showing that meditation practice improved sustained attention on the Continuous Performance Test (Rosvold et al., 1956). All of the above measures capture aspects of executive functioning (Barkley, 1997), thus providing robust evidence for the connection between meditation and executive function. However, the precise ‘mechanism’ behind this effect has not been sufficiently studied.
Executive control and the brain: the neural basis for performance monitoring
One of the most reliable neural markers of performance monitoring is the error-related negativity (ERN), an event-related potential that represents a neurophysiological response that is generated by the ACC (Dehaene et al., 1994) and that occurs within 100 ms of error commission (Falkenstein et al., 1990; Gehring et al., 1993). Though theorists agree that the ERN is implicated in executive control, there is a debate about its precise function. Specifically, there is disagreement about whether the ERN reflects a purely cognitive process or whether it also represents aspects of motivation and affect (Yeung, 2004; Inzlicht and Al-Khindi, in press).
Botvinick and colleagues (2001; Yeung et al., 2004) propose that the ERN represents conflict monitoring, so that when an error is made, the motor programs for both the correct and incorrect responses are co-activated, producing an ERN. According to this theory, negative-going waves like the ERN occur not only upon the commission of an error, but also upon correct responses that are high in conflict, such as incongruent trials on the Stroop task (Botvinick et al., 1999). Another computational model proposed by Holroyd and Coles (2002), casts the ERN as a marker of expectancy violation, being produced when the actual outcome (e.g. an error) differs from the expected outcome (e.g. a correct response). Holroyd and Coles (2002) explain that the ERN serves the function of a reinforcement learning signal, helping to adjust actual behavior closer to expected behavior.
More recent research investigating the ERN, however, suggests that the above models may not provide a complete account of the ERN. In particular, there exists a growing body of evidence to support the notion that the ERN, at least partially, reflects an index of motivational engagement and that the ERN may represent a distressed response that occurs when performance is worse than expected (see Weinberg et al., 2012). Since errors are usually associated with some degree of distress, as well as the physiological changes that accompany such distress (e.g. Critchley et al., 2003; Hajcak et al., 2003; Hajcak and Foti, 2008), it is not entirely surprising that the ERN has been found to be associated with negative affect (e.g. Luu et al., 2000, 2003; Bartholow et al., 2012). For example, studies have shown that patients with anxiety disorders exhibit a higher ERN than do healthy controls (Gehring et al., 2000). In addition, the ERN is diminished by anxiolytic drugs (Johannes et al., 2001), and is related to the defensive startle threat response (Hajcak and Foti, 2008). Furthermore, individuals have been found to exhibit heightened ERNs when the motivational salience of errors was manipulated through incentives (Hajcak et al., 2005; Ganushchak and Schiller, 2008) and setting accuracy goals (Gehring et al., 1993; Falkenstein et al., 2000). In sum, the ERN is related to executive control and attentional control, but also to affect and motivation. Given the nature of this event-related potential, we wondered whether exploring the link between meditation and the ERN could reveal ‘why’ meditation increases executive control—because of improved attention or because of improved motivational engagement.
Although the relation between meditation and the ERN was our main concern, we also wondered whether another ERP, the error positivity (Pe), could reveal why meditation leads to better executive control. The Pe is a later occurring component, seen after the ERN on error trials and is thought to represent the degree to which errors are ‘consciously’ detected (Hester et al., 2005). As such, we turned to this ERP to explore whether or not meditators display stronger conscious reactions to their errors in hopes of uncovering the process by which meditation practice improves executive control.
Meditation and the brain
In the quest to understand the processes underlying the palliative nature of meditation, researchers have turned to biological measures, and numerous studies have investigated the implementation of meditation practice in the brain using electroencephalogram (EEG) and fMRI (see Cahn and Polich, 2006, for a review). Given the importance of the ACC to executive function (Botvinick et al., 2004), we focus here on research exploring the impact of meditation on the ACC. Acting as one of the primary brain structures implicated in executive functioning, the ACC allows us to modify our behavior by comparing current behavior with an ideal desired outcome (Botvinick et al., 2001). This process is of particular relevance to meditation. Specifically, if one’s desired outcome is non-judgmental, mindful awareness, but one’s current behavior is rumination, the ACC facilitates modification of the current behavior, in order to achieve the desired goal (Kerns et al., 2004).
As such, many studies have attempted to examine the effects of meditation on the ACC. Interestingly, however, these have yielded mixed results. For example, one study found ACC deactivation in experienced Zen meditators during meditation (Ritskes et al., 2003), whereas another study reported increased activation in the rostral ACC during Vipassana meditation (Holzel et al., 2007). In addition, the ACC increases in activity during mantra meditation as compared with control (Lazar et al., 2000). In contrast to these findings, Brefczynski-Lewis et al., (2004) found ACC activation during meditation only in novice meditators, but not in experienced meditators. Furthermore, Tang and Posner (2009) documented increases in ACC activation during a resting condition that followed a period of integrative body and mind training. In sum, the role of the ACC in meditation practice is currently blurred.
As with all brain structures, there is no one-to-one relationship between the ACC and a specified function—the ACC is involved in many states and functions (e.g. Craig, 2009; Shackman et al., 2011)—thus it is difficult to know what to conclude from the above results. Therefore, our goal was not to examine ACC activity in meditators during actual practice, or periods of rest, but instead to examine how ACC-related executive control activity differs in experienced meditators compared with non-meditators. Specifically, we investigated the underlying processes that account for improved executive control among meditators by examining the manifestations of this relationship in the brain, and since there is currently little debate about the ACC’s importance for executive control, we turned to this region in specific to learn about how meditation improves executive functioning.
Overview and hypotheses
For the current experiment, experienced meditators and controls completed a Stroop task while we recorded ACC activity using an EEG. Since previous research has linked meditation expertise to improved executive functioning, and because the Stroop task is known to measure the inhibition facet of executive control, we hypothesized that meditators would exhibit better executive control (i.e. fewer Stroop errors) than would controls. We also predicted that meditators would exhibit higher amplitude ERNs in response to their errors, essentially facilitating improved performance. In predicting that, meditators would display higher ERNs, we wondered about the role that the two facets of mindfulness—present moment awareness and acceptance of emotional states—would play in this relationship. Specifically, we wondered if meditators’ superior ability to focus on the present or their ability to accept and embrace their emotions (as measured by the Philadelphia Mindfulness Scale) would predict better control and higher ERNs.
METHODS
Participants
In total, 44 participants from the community were recruited to participate in an EEG study, in exchange for $40. Meditators were recruited from meditation centers, as well as Craigslist (a classifieds website), whereas non-meditators were recruited from Craigslist exclusively. All meditators reported at least 1 year of meditation experience (M = 3.19, s.d. = 1.39), whereas non-meditators reported having no experience. Our meditators came from various meditation backgrounds (i.e. Vipassana, Shambhala, concentrative, etc). Although there are important differences among various meditation types, there are also many fundamental uniform elements that manifest in like outcomes for practitioners (Tang et al., 2010). In accordance with this research, we examined all meditators in the same analysis. We eliminated six participants from all analyses due to too few errors (<5) to calculate a reliable ERN (n = 1) (Olvet and Hajcak, 2009), equipment malfunction (n = 4) and excessive (>100) number of errors (n = 1). This left a total of 20 meditators (11 females, Mage = 33.00, s.d. = 11.49) and 18 non-meditators (16 females, Mage = 37.47, s.d. = 14.56) in the sample.
Individual difference measures
Prior to recording brain activity, all participants completed several demographic questionnaires (i.e. age, gender, level of education and socioeconomic status), as well as questions regarding how many years of meditation experience they had, and how many hours per week they currently spend meditating. Finally, participants completed both subscales of the 20-item Philadelphia Mindfulness Scale on a 5-point Likert scale (Cardaciotto et al., 2008). The Philadelphia Mindfulness Scale measures present moment awareness (e.g. I am aware of what thoughts are passing through my mind), which is typically positively correlated with attention and reflection, and emotional acceptance (e.g. I try to distract myself when I feel unpleasant emotions), which is typically negatively correlated with rumination and thought suppression.
Procedure
After providing demographic information, participants completed a Stroop task, a measure of executive control. This task consisted of a series of color words (red, green, blue or yellow), each of which was presented in a color that either matched (congruent) or did not match (incongruent) the semantic meaning of the word. Participants were instructed to identify the color in which each word was presented by pressing the corresponding colored button on a response box. Each trial consisted of a fixation cross (‘+’) presented for 500 ms, followed by the stimulus word presented for 200 ms, and a response window of 1000 ms. The inter-trial interval was 1000 ms. Participants completed 10 blocks, each consisting of 32 congruent trials and 16 incongruent trials. We calculated a Stroop incongruency effect (reaction times on incongruent trials minus reaction times on congruent trials; only looking at correct trials) and tallied the number of errors.
Neurophysiological recording and processing
EEG activity during the Stroop task was recorded using a stretch Lycra cap embedded with 32 tin electrodes. Recordings were digitized at 512 Hz using ASA acquisition software (Advanced Neuro Technology B.V., Enschede, The Netherlands) with average-ear reference and forehead ground. EEG data was corrected for vertical electro-oculogram artifacts (Gratton et al., 1983) and digitally filtered offline between 1 and 15 Hz (FFT implemented, 24 dB, zero phase-shift Butterworth filter). We based our filter parameters on previously published work (e.g. Inzlicht and Gutsell, 2007; Bartholow et al., 2012).1 The period between 200 and 0 ms before key press was used for baseline correction. Epochs were defined between 200 ms before and 800 ms after the response for artifact-free trials. Data for these epochs were averaged within participants separately for correct and incorrect trials. The ERN was defined as the minimum deflection between 50 ms before and 150 ms after the key press at the frontocentral midline electrode (FCz), while the Pe was defined as the maximum peak between 150 and 250 ms postkey press at FCz electrode. The ERN and Pe were calculated by averaging maximum negativities and positivities across all incorrect trials, respectively. All error trials (i.e. both congruent and incongruent) were used when calculating the ERN and Pe. Although including all error trials allows for the possibility that not all analyzed errors represent the ability to inhibit pre-potent responses, we felt that examining all errors would provide us with a clearer picture of neural performance monitoring.
RESULTS
Meditation experience
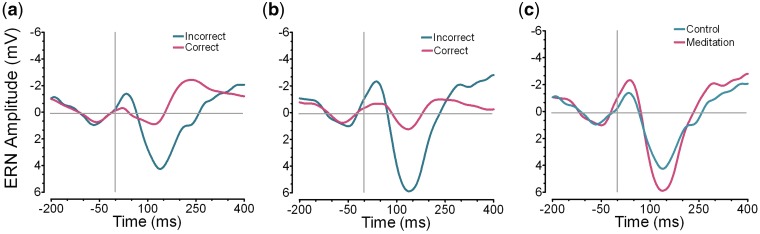
We hypothesized that meditators would exhibit a higher ERN than non-meditators. Given our directional prediction and voluminous past research linking meditation and mindfulness with markers of executive control, all analyses are one-way. Figure 1 illustrates that meditators did indeed have higher amplitude ERNs (M = −3.49, s.d. = 2.12), than non-meditators (M = −2.26, s.d. = 2.01), F(1, 36) = 3.32, P < 0.04, d = 0.58. We next examined the effect of meditation practice continuously by looking at years and hours of meditation experience. This analysis proved more robust. Due to software malfunction, we failed to record the meditation practice experience for two participants and replaced these values with the series mean.2 Given that half the sample (control participants) had no meditation experience, the distribution of meditation experience is non-normal and Pearson correlation coefficients may not be appropriate. Therefore, in order to correct for violations of normality, we conducted bootstrap analyses with 5000 samples for all analyses conducted with years and hours of meditation, and report the 95% confidence intervals (95% CIs) looking to see if these intervals include 0; we also report Pearson correlations for completeness. Results revealed that years of meditation experience significantly predicted ERN amplitude, (95% CI −0.65 to −0.02), r (37) = −0.37, P < 0.02, d = 0.80, as did meditation frequency, (95% CI −0.58 to −0.06), r (37) = −0.35, P < 0.02, d = 0.75 (Table 1). Importantly, the effect of meditation experience and frequency on the ERN held constant when controlling for age, gender, education and socioeconomic status, all P’s < 0.03.This confirms that meditation practice boosted neurophysiological response to errors.
Fig. 1.
ERPs at electrode FCz in the (a) control and (b) meditation conditions on correct and incorrect trials and (c) the ERN on incorrect trials for participants in the two conditions.
Table 1.
Bivariate correlations for main study variables.
| Years meditating | Hours per week | ERN | Pe | Emotional acceptance | Mindful awareness | Total errors | Stroop effect | |
|---|---|---|---|---|---|---|---|---|
| Years meditating | 0.79*** (0.66 to 0.92) | −0.37** (−0.65 to −0.01) | −0.22 (−0.59 to 0.05) | 0.55*** (0.28 to 0.74) | 0.27* (−0.12 to 0.18) | −0.27* (−0.45 to −0.02) | −0.04 (−.28 to 0.30) | |
| Hours per week | −0.35* (−0.58 to −0.05) | −0.21 (−0.57 to 0.08) | 0.39** (0.11 to 0.62) | 0.18 (−0.17 to 0.51) | −0.23 (−0.42 to 0.05) | −0.06 (−0.26 to 0.21) | ||
| ERN | 0.20 (−0.09 to 0.45) | −0.31* (−0.57 to −0.03) | −0.01 (−0.40 to 0.42) | 0.25 (−0.18 to 0.48) | −0.08 (−0.48 to 0.27) | |||
| Pe | −0.01 (−0.40 to 0.30) | −0.25 (−0.48 to 0.03) | 0.17 (−0.10 to 0.42) | 0.25 (−0.06 to 0.51) | ||||
| Mindful acceptance | 0.29* (0.08 to 0.59) | 0.32* (−0.52 to −0.09) | 0.06 (−0.23 to 0.46) | |||||
| Mindful awareness | −0.09 (−0.30 to 0.13) | 0.12 (−0.51 to 0.38) | ||||||
| Total errors | −0.12 (−0.31 to 0.28) | |||||||
| Stroop effect |
Confidence intervals for bootstrap analyses are presented in parentheses. n = 38 and n = 37 for all analyses with ‘total errors’ and ‘stroop effect’.
***P < 0.001; **P ≤ 0.01; *P < 0.055.
Next, we wanted to test whether or not meditators exhibit greater Pe’s than do controls. It turns out that meditators did not exhibit significantly greater Pe’s (M = −2.32, s.d. = 2.28) than did controls (M = −1.36, s.d. = 2.35), F(1, 36) = 1.59, P > 0.10. In addition, neither meditation experience, nor meditation frequency significantly predicted Pe amplitude, P’s > 0.10. Given the lack of a basic effect with the Pe, we no longer consider it in subsequent analyses.
Since the ERN often correlates with performance on executive control tasks (Yeung, 2004; however, see Weinberg et al., 2012), it is important to examine ERN effects controlling for indices of performance. Thus, in order to eliminate any performance confounds between groups, we examined the effects of meditation experience and frequency on the ERN while controlling for total number of errors and overall reaction time. Results revealed that years meditating still predicted ERN amplitude when controlling for the number of error trials and reaction times, P < 0.02; similarly, meditation frequency predicted the ERN when controlling for number of errors and reaction time, P < 0.025. This excludes the possibility that meditators displayed higher ERNs simply because they performed better than controls did. It suggests, in other words, that our effect was not some epiphenomenon of cognitive performance
Mindfulness
Next, we tested for any associations between group, meditation experience, self-reported mindfulness and the ERN. As expected, group (meditator vs control) significantly predicted emotional acceptance, F(1, 36) = 6.67, P < 0.01, d = 0.86, with meditators reporting significantly higher levels of acceptance, M = 3.52, s.d. = 0.81, than non-meditators, M = 2.93, s.d. = 0.56. Surprisingly, condition did not predict mindful awareness, F(1, 36) = 1.26, ns, with meditators, M = 4.02, s.d. = 0.10, reporting similar present moment awareness as non-meditators, M = 3.89, s.d. = 0.11. Similarly, years meditating was significantly associated with mindful acceptance, (95% CI 0.28–0.74), r (37) = 0.55, P < 0.001, d = 1.32 as was meditation frequency, (95% CI 0.11–0.63), r (37) = 0.40, P < 0.01, d = 0.87. The relationship between years meditating and awareness, (95% CI −0.11 to 0.58), r (37) = 0.27, P = 0.05, d = 0.56 and between meditation frequency and awareness, (95% CI −0.17 to 0.50), r (37) = 0.18, ns, were less robust (Table 1). These associations are particularly intriguing because they suggest that meditation practice may be more important in influencing emotional acceptance than in influencing present moment awareness.
Next, we examined the association between mindfulness and the ERN. As predicted, mindful acceptance was correlated with ERN amplitude (95% CI −0.57 to −0.02), r (37) = 0.31, P < 0.03, d = 0.65, suggesting that individuals with higher emotional acceptance displayed higher ERNs. Figure 2 displays average ERNs for all incorrect trials among participants ranking high and low on mindful acceptance, as determined by a median split. When controlling for total errors and reaction time, the association between acceptance and the ERN became marginal (95% CI −1.54 to 0.06), P = 0.054. Mindful awareness, in contrast was not significantly correlated with the ERN (95% CI −0.40 to 0.42), r (37) = −0.01, ns.
Fig. 2.
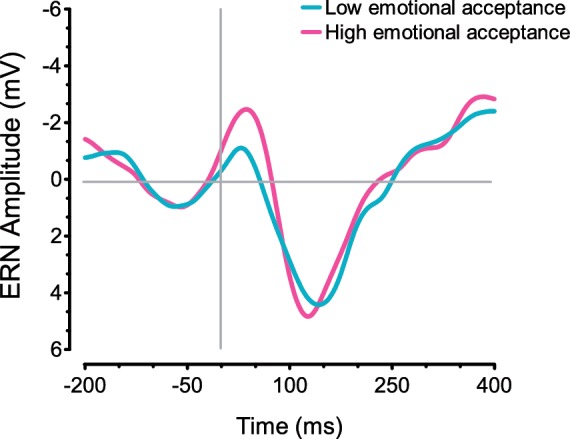
The ERN on incorrect trials for participants high and low on mindful acceptance, as determined by a median split.
STROOP TASK PERFORMANCE
Finally, we examined the effect of meditation practice on executive control, as measured by Stroop performance, specifically errors on the Stroop task. One participant was excluded from all analyses because he/she was an extreme outlier, ESD = 4.37, P < 0.05. As predicted, meditators made fewer Stroop errors, M = 16.05, s.d. = 6.37, than controls, M = 22.66, s.d. = 19.95, although this trend was not robust, t(20, 27) = −1.55, P = 0.069, d = 0.69 (equal variance not assumed). We found more robust results for years meditating (95% CI −0.45 to −0.02), r (36) = −0.27, P = 0.054, d = 0.56; but those that were less strong for meditation frequency (95% CI −0.43 to 0.04), r (36) = −0.23, P = 0.082, d = 0.47. The association between mindful acceptance and Stroop error rate was stronger, (95% CI −0.51 to −0.10), r(36) = −0.32, P < 0.03, d = 0.68, suggesting that the ability to mindfully accept emotional states relates to executive control. We also ran the above analysis controlling for reaction time in order to account for potential speed–accuracy tradeoffs. Results of these analyses revealed that the strength of the relationship between acceptance and total errors did not change, even when controlling for reaction times, P < 0.035, eliminating the possibility that individuals high in emotional acceptance performed better on the Stroop task simply because they took more time to respond. Paralleling the analyses with the ERN, mindful awareness was not associated with the number of Stroop errors (95% CI −0.31 to 0.14), r (36) = −0.09, ns. Finally, we examined the effect of meditation experience, meditation frequency, mindful awareness and emotional acceptance on the Stroop incongruency effect. However, these analyses proved to be non-significant, all P’s > 0.10.
Process: from meditation experience to improved executive control
Finally, we tested the mediating effect of both emotional acceptance and the ERN on the link between meditation experience and improved executive control. Not only did we want to examine two mediators at once, we also wanted to test the interactive effects of the two mediators on each other. Therefore, a test of multiple mediation was performed using the SPSS modeling macro procedure, MED3C, outlined by Hayes et al. (2011). This multiple mediation procedure offered the advantage of testing two mediators simultaneously (i.e. improved emotional acceptance and increased ERN amplitude) rather than separately, in order to determine the overall effect of both mediators, as well as to obtain a clearer picture of the unique effects of each mediator (see L. Inzlicht and M. Inzlicht, Submitted for publication). The total, direct and indirect effects of condition on performance were estimated using a set of OLS regressions. To ascertain indirect effects, percentile-based bootstrap confidence intervals and bootstrap estimates of standard errors were generated based on 1000 bootstrap samples.
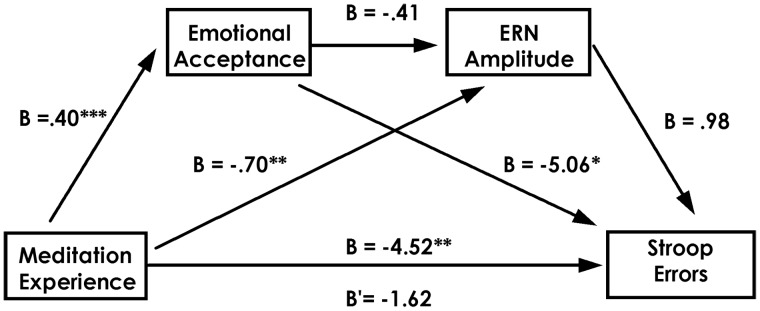
We calculated our independent variable, meditation experience, by standardizing, then averaging years-meditating and meditation frequency. Given the theoretical association between reaction time and error rate, and between age and years meditating, we used average reaction time and age as covariates in our analysis. As outlined above, meditation experience predicted fewer Stroop errors, t(36) = −1.69, P = 0.05, more emotional acceptance, t(36) = 3.35, P < 0.01, and more negative ERN amplitudes, t(36) = −1.64, P = 0.055. Importantly, when we entered both mediators into the analysis, the association between meditation experience and Stroop performance dropped from significance, t(36) = −0.51, ns. We tested for the significance of this effect using the bootstrap method. This analysis revealed that the unique indirect effect of emotional acceptance on Stroop performance was significant, estimate = −2.04, (95% CI −5.24 to −0.03), s.e. = 1.42. This suggests that emotional acceptance mediates the link between meditation experience executive control. Furthermore, although the unique indirect effects of ERN amplitude on performance or between the combination of ERN amplitude and emotional acceptance was not significant, the total indirect effect of all mediation paths (i.e. emotional acceptance, ERN and combined emotional acceptance and ERN) on Stroop performance was significant, estimate = −2.90, (95% CI −7.79 to −0.09), s.e. = 2.10. These findings, highlighted in Figure 3, suggest that meditation increases performance on the Stroop primarily through heightened emotional acceptance, and to a lesser degree through enhanced neural signals of self-control errors. The same set of analyses conducted with mindful awareness produced non-significant results.
Fig. 3.
The mediating role of emotional acceptance and ERN amplitude in the link between meditation experience and Stroop performance (errors). Unstandardized regression coefficients are presented. The analysis uses average reaction time and age as covariates. ***P < 0.01; **P ≤ 0.055; *P < 0.10.
DISCUSSION
The results of the current study confirm that meditation is related to better executive control, but further suggest that this effect is implemented in the ACC as indexed by an amplification of the ERN, a neural signal of error processing. Furthermore, this work suggests that enhanced acceptance of emotional states may be a key reason that meditation improves executive functioning. Though meditators are typically known to be expert emotional regulators (Perlman et al., 2010), it is also the case that meditators are highly attuned to their emotions (Teasdale et al., 2002; Niemiec et al., 2010). In other words, they identify their emotions quickly and accurately. Particularly good evidence to this effect is a study that found a negative association between trait mindfulness and alexithymia, a clinical disorder characterized by the inability to recognize one’s own emotions (Baer et al., 2006). Pilot research from our laboratory confirms these results.3 If meditation experience results in enhanced attention to the emotions associated with making errors, it is not surprising that this emotional attunement would translate to improved executive functioning. It is also interesting to note that the present moment attention facet of mindfulness did not significantly correlate with the ERN, suggesting that the executive control benefits of meditation are more related to affect than attention; this also confirms the important affective component to the ERN.
Another interesting finding is that although meditators exhibit stronger ERNs in response to their errors, they do not exhibit stronger Pe’s. The Pe is thought to represent a conscious reaction to errors, suggesting that although meditators quickly react to their errors, as reflected by a higher amplitude ERN, they are also quick to let go of any reaction associated with them. These results seem compatible with mindfulness theory (Williams, 2010), as well as past research on meditators’ emotional reactivity (Goldin and Gross, 2010).
Limitations
Though the results of the current experiment suggest that meditation practice leads to enhanced control by enhancing emotional acceptance, more work is needed to clarify the relationship between meditation and emotional acceptance. Since our study did not employ a direct measure of emotional sensitivity, it is difficult to say whether meditators experience sharper affective pangs upon making errors, consequently resulting in improved performance or whether they are simply more attuned to those pangs. Future studies would benefit from exploring this issue in more depth.
Another issue worth noting is the diverse meditation backgrounds that our group of meditators consisted of. Although there are numerous important elements that are consistent among all schools of meditation, there are also crucial differences that exist. While the results of our study suggest that even individuals practicing different forms of meditation all show higher ERNs, emotional acceptance and executive control, future research is needed to confirm that these effects remain when specific types of meditation are studied separately.
CONCLUSION
The results of previous meditation research suggest that meditation improves executive functioning. The results of our study confirm this finding, but further extend it to suggest that this effect can be accounted for by an increase in the acceptance of emotional states, as well as the neural basis for performance monitoring. In other words, meditators may excel at executive control because of their ability to attend to the emotions associated with making errors—a process implemented in the ACC. Specifically, if emotional acceptance is associated with an increase in error-related neural activity, it is not surprising that meditation practice improves control. These findings shine new light on the effect of meditation practice and mindfulness on executive functioning, suggesting that this relationship may not be purely cognitive in nature. This new focus on the role of emotionality may be an important one for future studies that wish to explore the relationship between meditation, mindfulness and executive control in greater depth.
FUNDING
Funding for this research was provided by grants to Rimma Teper and Michael Inzlicht from the Social Sciences and Humanities Research Council.
Conflict of Interest
None declared.
Acknowledgments
We thank Lisa Legault, Elizabeth Page-Gould, Kirk Brown, Alexa Tullet, Jennifer Gutsell, Sonia Kang, Jacob Hirsh and Shona Tritt for valuable insights. We also thank Rodrigo Achala, Emerson Lai, Geith Maal-Bared, Marina Rain, Adriana Salcedo and Man-On Tong for their assistance with data collection.
Footnotes
1Luck (2005) has recommended that only modest high-pass filters (e.g. 0.1 or 0.5 Hz) should be used for fear that they could change the morphology and latency of ERPs. However, in past studies (Inzlicht and Al-Khindi, in press) we have found that filtering at lower (0.1 Hz) or higher (1 Hz) frequencies yield practically identical results for peak amplitude analyses of the ERN, r(36) = 0.82, P < 0.01.
2Due to equipment malfunction, we were missing data from several participants for several variables; specifically meditation experience (n = 2), Stroop performance (n = 1) and the Phildelphia Mindfulness Scale (n = 2). These missing data points were replaced with the series mean.
3In a pilot sample of 22 participants, we find that mindful acceptance is highly negatively correlated with alexithymia as measured by the Toronto Alexithymia Scale (Bagby et al., 1994), r(21) = −0.80, P < 0.001. This suggests that the acceptance facet of the Philadelphia Mindfulness Scale (Cardaciotto et al., 2008) relates to the ability to describe and identify one’s feeling states.
REFERENCES
- Baer RA, Hopkins J, Krietemeyer J, Smith GT, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JDA, Taylor GJ. The twenty-item Toronto Alexithymia Scale-I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Henry EA, Lust SA, Saults JS, Wood PK. Alcohol effects on performance monitoring and adjustment: Affect modulation and impairment of evaluative cognitive control. Journal of Abnormal Psychology. 2012;121:173–86. doi: 10.1037/a0023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Davidson RJ. A neural correlate of attentional expertise in long-term Buddhist practitioners. The 2004 Annual Meeting for the Society for Social Neuroscience; San Diego, CA. 2004. [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology. 2003;84:822–48. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Cardaciotto L, Herbert JD, Forman EM, Moitra E, Farrow V. The assessment of present-moment awareness and acceptance: The Philadelphia Mindfulness Scale. Assessment. 2008;15:204–23. doi: 10.1177/1073191107311467. [DOI] [PubMed] [Google Scholar]
- Chambers R, Lo C, Allen NB. The impact of intensive mindfulness training on executive cognition, cognitive style, and affect. Cognitive Therapy and Research. 2008;32:303–22. [Google Scholar]
- Compton RJ, Robinson MD, Ode S, Quandt LC, Fineman SL, Carp J. Error-monitoring ability predicts daily stress regulation. Psychological Science. 2008;19:702–8. doi: 10.1111/j.1467-9280.2008.02145.x. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, et al. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126:2139–52. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–5. [Google Scholar]
- Deikman AJ. De-automatization and the mystic experience. Psychiatry. 1966;29:324–38. doi: 10.1080/00332747.1966.11023476. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Brunia CHM, Gailard WK, Kok A, editors. Psychophysiological Brain Research. Vol. 1. Tilburg, the Netherlands: Tilburg University Press; 1990. pp. 192–5. [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–7. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Ganushchak LY, Schiller NO. Motivation and semantic context affect brain error monitoring activity: an event-related brain potentials study. Neuroimage. 2008;39:395–405. doi: 10.1016/j.neuroimage.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–90. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive–compulsive disorder. Psychological Science. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Goldin P, Gross J. Effect of mindfulness meditation training on the neural bases of emotion regulation in social anxiety disorder. Emotion. 2010;10:83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neuropsychology. 1983;55:468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Foti D. Errors are aversive: defensive motivation and the error-related negativity. Psychological Science. 2008;19:103–8. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: Error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40:895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–60. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Preacher KJ, Myers TA. Mediation and the estimation of indirect effects in political communication research. In: Bucy EP, Lance Holbert R, editors. Sourcebook for Political Communication Research: Methods, Measures, and Analytical Techniques. New York: Routledge; 2011. [Google Scholar]
- Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage. 2005;27:602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Hempel H, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neuroscience Letters. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Al-Khindi T. ERN and the placebo: a misattribution approach to studying the arousal properties of the error-related negativity. Journal of Experimental Psychology: General. in press doi: 10.1037/a0027586. doi: 10.1037/a0027586. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Gutsell JN. Running on empty: neural signals for self-control failure. Psychological Science. 2007;18:933–7. doi: 10.1111/j.1467-9280.2007.02004.x. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive Affective and Behavioral Neuroscience. 2007;7:109–19. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Dengler R, Munte TF. Oxazepam alters action monitoring. Psychopharmacology. 2001;155:100–6. doi: 10.1007/s002130100680. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain and Illness. New York: Dell; 1990. [Google Scholar]
- Kabat-Zinn J. Wherever You Go, There You Are: Mindfulness Meditation in Everyday Life. New York: Hyperion; 1994. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate, conflict monitoring, and adjustments in control. Science. 2004;303:1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Bush G, Gollub RL, et al. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11:1581–5. [PubMed] [Google Scholar]
- Luck S. An Introduction to the Event-Related Potential Technique. Cambridge: MIT Press; 2005. [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiologic responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Kristeller JL. Mindfulness and meditation. In: Miller EW, editor. Integrating Spirituality Into Treatment: Resources for Practitioners. Washington, DC: American Psychological Association; 1999. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A. The unity and diversity of executive functions and their contributions to complex “frontal lobel” tasks: a latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moore A, Malinowski P. Meditation, mindfulness & cognitive flexibility. Consciousness and Cognition. 2009;18:176–86. doi: 10.1016/j.concog.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Niemiec CP, Brown KW, Kashdan TB, Cozzolino PJ, Breen W, Levesque C, Ryan RM. Being present in the face of existential threat: The role of trait mindfulness in reducing defensive responses to mortality salience. Journal of Personality and Social Psychology. 2010;99:344–65. doi: 10.1037/a0019388. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46:957–61. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Perlman DM, Salomons TV, Davidson RJ, Lutz A. Differential effects on pain intensity and unpleasantness of two meditation practices. Emotion. 2010;10:65–71. doi: 10.1037/a0018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritskes R, Ritskes-Hoitinga M, Stodkilde-Jorgensen H, Baerentsen K, Hartman T. MRI scanning during Zen meditation: The picture of enlightenment? Constructivism in the Human Sciences. 2003;8:85–90. [Google Scholar]
- Rosvold H, Mirsky F, Sarason I, Bransome E, Beck L. A continuous performance test for brain damage. Journal of Consulting Psychology. 1956;20:343–50. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel BJ, Vohs KD, Baumeister RF. Intellectual performance and ego depletion: Role of the self in logical reasoning and other information processing. Journal of Personality and Social Psychology. 2003;85:33–46. doi: 10.1037/0022-3514.85.1.33. [DOI] [PubMed] [Google Scholar]
- Semple RJ. Does mindfulness meditation enhance attention? A randomized controlled trial. Mindfulness. 2010;1:121–30. [Google Scholar]
- Stanley EA, Schaldach JM, Kiyonaga A, Jha AP. Mindfulness-based mind fitness training: A case study of a high-stress predeployment military cohort. Cognitive and Behavioral Practice. 2011;18:566–576. [Google Scholar]
- Tang Y, Lu Q, Geng X, Stein EA, Yang Y, Posner MI. Short-term meditation induces white matter changes in the anterior cingulate. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(35):15649–52. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Ma Y, Wang J, et al. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17152–6. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Posner MI. Attention training and attention state training. Trends in Cognitive Neuroscience. 2009;13:222–7. doi: 10.1016/j.tics.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Moore RG, Hayhurst H, Pope M, Williams S, Segal ZV. Metacognitive awareness and prevention of relapse in depression: Empirical evidence. Journal of Consulting and Clinical Psychology. 2002;70:275–87. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- Vohs KD, Baumeister RF, Ciarocco NJ. Self-presentation: Regulatory resource depletion impairs impression management and effortful self-presentation depletes regulatory resources. Journal of Personality and Social Psychology. 2005;88:632–57. doi: 10.1037/0022-3514.88.4.632. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, Hajcak G. Integrating multiple perspectives on error-related brain activity: The ERN as a neurobehavioral trait. Motivation and Emotion. 2012;36:84–100. [Google Scholar]
- Wenk-Sormaz H. Meditation can reduce habitual responding. Alternative Therapies in Health and Medicine. 2005;11:42–58. [PubMed] [Google Scholar]
- Williams JM. Mindfulness and psychological process. Emotion. 2010;10:1–7. doi: 10.1037/a0018360. [DOI] [PubMed] [Google Scholar]
- Yeung N. Relating cognitive and aff ctive theories of the error-related negativity. In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts, and the Brain: Current Opinions on Performance Monitoring. Leipzig: Max Planck Institute of Cognitive Neuroscience; 2004. pp. 63–70. [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–59. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]