Abstract
One component of mindfulness training (MT) is the development of interoceptive attention (IA) to visceral bodily sensations, facilitated through daily practices such as breath monitoring. Using functional magnetic resonance imaging (fMRI), we examined experience-dependent functional plasticity in accessing interoceptive representations by comparing graduates of a Mindfulness-Based Stress Reduction course to a waitlisted control group. IA to respiratory sensations was contrasted against two visual tasks, controlling for attentional requirements non-specific to IA such as maintaining sensation and suppressing distraction. In anatomically partitioned analyses of insula activity, MT predicted greater IA-related activity in anterior dysgranular insula regions, consistent with greater integration of interoceptive sensation with external context. MT also predicted decreased recruitment of the dorsomedial prefrontal cortex (DMPFC) during IA, and altered functional connectivity between the DMPFC and the posterior insula, putative primary interoceptive cortex. Furthermore, meditation practice compliance predicted greater posterior insula and reduced visual pathway recruitment during IA. These findings suggest that interoceptive training modulates task-specific cortical recruitment, analogous to training-related plasticity observed in the external senses. Further, DMPFC modulation of IA networks may be an important mechanism by which MT alters information processing in the brain, increasing the contribution of interoception to perceptual experience.
Keywords: interoception, fMRI, mindfulness, attention, insula, plasticity
INTRODUCTION
The psychologist Maslow (1943) famously argued that human behavior is motivated by a hierarchy of needs, ranking the body’s physiological requirements ahead of more abstract goals such as freedom, companionship or social status. Maslow’s hierarchy points to the complex interplay between two distinct representational systems for human attention: in cases of physiological imbalance, interoceptive attention (IA) is recruited to alert the individual to the body’s internal requirements (Liotti et al., 2001). When these needs are met, exteroceptive attention (EA) fosters exploratory behavior which aids in the pursuit of more conceptual goals (Gibson, 1988). In many cases, this transition from IA to EA is a natural and adaptive part of development, leading to a balanced sense of well-being as an individual becomes integrated with the social world (Ryan and Deci, 2000). However, placing a high importance on external goals can be problematic in the face of failure (Moberly and Watkins, 2010), as these events become diagnostic of an individual’s sense of self worth. In such situations, it may be difficult to disengage from patterns of negative cognitive elaboration that have become automatic and seemingly obligatory (Joorman and Siemer, 2011).
Mindfulness training (MT) may be one means by which to alter the relationship between external events and self-attribution, limiting automatic self-evaluative processing (Frewen et al., 2008). MT often begins through courses such as Mindfulness-Based Stress Reduction (MBSR) (Kabat-Zinn, 1990), in which individuals learn meditation techniques in a weekly group setting, and then practice at home with guided meditation and yoga audio recordings. There have been several accounts of the cognitive mechanisms by which MT promotes salutary effects, such as decentering of experience (Fresco et al., 2007), a broadened context for appraisal (Garland et al., 2011), or otherwise ‘reperceiving’ the world (Carmody et al., 2009). Despite progress in refining these cognitive models explaining higher-level effects of MT, we lack a translational account for how mindfulness practices directly modulate attention networks to promote cognitive change.
We propose that the development of IA may be one foundation by which MT promotes cognitive change. Many mindfulness practices involve sustained attention to interoceptive sensations of respiration or bodily sensation, designed to improve the stability and frequency with which one perceives the transitory nature of human experience (Kabat-Zinn, 1982; Baer et al., 2006; Brown et al., 2007; Ivanovski and Malhi, 2007). While some exercises in MBSR investigate emotional reactivity to external events, relying on EA to recognize one’s behavioral patterns in the world, most MBSR practices employ IA, cultivating sustained attention toward bodily sensation in response to stress. It may be this ability to skillfully recruit IA that disrupts automatic conceptual elaboration and allows for more adaptive regulatory strategies to be invoked. Supporting this notion, in prior work we have demonstrated that MBSR reduces the involuntary recruitment of a cognitive elaboration network, instead promoting recruitment of viscerosomatic regions associated with momentary awareness of internal sensation (Farb et al., 2007), and that this improved access to body sensation during sadness is associated with lower levels of depression (Farb et al., 2010). In the present study, we investigated whether practicing sustained IA through an MBSR course modulates neural representation networks for interoception.
It has long been recognized that sensory afferents terminate in specialized regions, cortical ‘maps’ that are sensitive to perturbations of sensory receptors from stimuli both inside and outside of the body (Kaas, 1987). Human perceptual acuity appears to be a flexible capacity that can be improved through training (Gibson, 1953), presumably modulating these cortical maps and surrounding representational cortices, although the biological mechanisms for such neuroplasticity are still being investigated (Barnes and Finnerty, 2010). Neuroplasticity research has focused predominantly on attention to external stimuli: EA training appears to alter task-evoked activity in domain-specific sensory cortices, particularly in vision (Kourtzi et al., 2005; Yotsumoto and Watanabe, 2008; Sasaki et al., 2010), although neural plasticity has been observed following training in other exteroceptive modalities, such as audition (Jancke et al., 2001), somatosensation (Hamilton and Pascual-Leone, 1998; Godde et al., 2003), taste (Faurion et al., 1998) and olfaction (Gottfried et al., 2002). A well-characterized, lateral frontoparietal network appears to be responsible for modulating recruitment of primary representation cortices in EA (Corbetta, 1998; Corbetta and Shulman, 2002; He et al., 2007; Vincent et al., 2008). However, not all senses rely equally upon this frontoparietal attention network, with the gustatory senses (taste and olfaction) demonstrating limbic responses compared to prefrontal responses to visual stimuli (Hurliman et al., 2005). Thus, there is reason to believe that IA may rely upon distinct attentional networks in addition to having its own primary representation cortices.
Distinct from even the gustatory senses, interoception involves sensation of the body itself, integrating visceral afferents associated with internal systems such as digestion, circulation, proprioception and respiration. Anatomical evidence suggests that that a lamina I spinothalamocortical pathway carries sympathetic afferents that signal the physiological condition of all tissues of the body (Craig, 2002). By way of the brainstem parabrachial nucleus and ventromedial thalamus, this pathway projects to the posterior granular and middle dysgranular regions of the insular cortex, serving as primary interoceptive cortex (Flynn, 1999), analogous to primary visual cortex in the occipital lobe or primary auditory cortex in the superior temporal gyrus. Much as visual awareness requires integration between occipital cortices and prefrontal cortical regions (Vanni et al., 1996), interoceptive representations may be refined and filtered for contextual relevance. Sensory signals propagate forward toward the prefrontal cortex through the anterior dysgranular insula, dorsal aspects of the anterior insula that integrate afferent physiological signals with higher-order contextual information (Damasio et al., 2000; Critchley, 2005; Craig, 2009; Mutschler et al., 2009). While the posterior insula may constitute a primary interoceptive region, the anterior insula appears to integrate internal and external signals, regulating the direction of external attention both in constructive biases such as empathy (Singer et al., 2009) and maladaptive biases such as addiction (Naqvi and Bechara, 2009). Thus, while there are strong anatomical connections between the insula’s posterior interoceptive regions and its anterior zones (Chikama et al., 1997), it is likely that the anterior regions are also heavily influenced by attention to external stimuli. Critically, the dominance of IA or EA in promoting anterior insula activity may be influenced by the attentional habits of the individuals being investigated, such as how anxious individuals recruit the anterior insula more robustly than healthy controls during interoceptive monitoring (Critchley et al., 2004; Paulus and Stein, 2006).
The propagation of interoceptive signals from the posterior to anterior insula makes it an intriguing candidate mechanism for investigating training-related plasticity in interoceptive representation. Behaviorally, training appears to improve interoceptive accuracy for tasks such as heartbeat detection (Brener and Jones, 1974), suggesting possible neuroplasticity in an interoceptive representation network. Indeed, interoceptive practice through meditation programs such as MBSR may alter brain structure: meditation has been linked to increased gray matter volume in sensory regions such as the insula and somatosensory cortex, parietal attentional regions and paralimbic regions such as the hippocampus and inferior temporal gyrus (Lazar et al., 2005; Holzel et al., 2008, 2011). A similar functional network, including the right insula in particular, appears to be more powerfully activated following MBSR during the deployment of mindful attention (Farb et al., 2007). These findings suggest that MT may increase the capacity for the sustained encoding of interoceptive sensation in regions such as the anterior insula and other paralimbic regions.
In the present study, we used functional magnetic resonance imaging (fMRI) to examine the effects of MT on the cortical representation of IA. We contrasted an untrained, waitlisted control group against individuals who had recently completed the 8 week MBSR training program. To measure IA recruitment, neural activity associated with breath monitoring was contrasted against two visual EA tasks, controlling for the common attentional requirements of maintaining sensory awareness and suppressing distraction (Bunge et al., 2001). Using this paradigm, we were able to evaluate whether the representation of IA, including its specific propagation through the insula, was altered as a function of MT.
MATERIALS AND METHODS
Participants
Participants were recruited upon enrollment in the Mindfulness-Based Stress Reduction (MBSR) program at St. Joseph’s Hospital in Toronto, and randomly assigned to the training (MT) or waitlisted conditions (untrained). The untrained group included 12 women and 4 men (N = 16; mean age 42.00 ± 9.24), while the MT group included 15 women and 5 men (N = 20; mean age 45.55 ± 13.38). The two groups did not differ in terms of mean age [t(34) = 0.90, n.s.] nor in terms of gender distribution [ = 1, n.s.]. All participants were right-handed volunteers that gave informed consent to procedures approved by the Sunnybrook and Women’s College Health Sciences Clinical Ethics Committee. While all participants lived independently in the community and were screened for suicidal ideation, substance abuse and mental health problems that would preclude course participation, many participants reported high levels of stress and dysphoric affect (for more symptom information, please see Farb et al., 2010).
= 1, n.s.]. All participants were right-handed volunteers that gave informed consent to procedures approved by the Sunnybrook and Women’s College Health Sciences Clinical Ethics Committee. While all participants lived independently in the community and were screened for suicidal ideation, substance abuse and mental health problems that would preclude course participation, many participants reported high levels of stress and dysphoric affect (for more symptom information, please see Farb et al., 2010).
MBSR training procedure
The MBSR course introduced participants to the practice of moment-to-moment, non-judgmental awareness through an 8 week program. Participants attended weekly group sessions introducing them to formal mediation practices, gentle yoga and education on stress responses and management. The course also included informal diary exercises later discussed in the group setting: in 1 week, participants focused on positive events, another week, on negative events and another week on monitoring stressful communications. However, common to even these externalized practices, there was an emphasis on how these events occurred from an interoceptive perspective, i.e. how one felt in one’s body during these events. The MBSR program also included a full day of silent meditation between the 6th and 7th meeting sessions. Participants were required to attend at least seven of the eight group sessions and the full day session to be considered compliant with the training protocol. In addition to group meetings, on non-class days participants were asked to practice yoga and/or meditation for ∼40 min a day with the assistance of guided meditation CDs, and to perform weekly reflection and diarizing exercises. The formal meditation practices included breath monitoring, body scans (the progressive direction of attention to different parts of the body) and diffuse direction of attention to sounds, thoughts, feelings and bodily sensations.
MBSR practice compliance was operationalized as the percentage of time practiced on non-meeting days given an assigned time of 40 min, the length of a guided meditation CD. Participants were instructed to maintain a daily log of practice completion, which was collected by the course instructors at the end of the course. Participants were required to complete at least 50% of the recommended daily homework to be eligible for the study. Of the 23 participants originally enrolled in the MBSR group, 20 were retained for the current study, whereas the other 3 did not meet course participation requirements. Using a 3:2 randomization ratio, a greater proportion of participants were randomized to the MBSR group (23 vs 16) with the knowledge that there would be some participant dropout before the MT group scans could be acquired. Untrained participants were scanned within the 4 weeks prior to beginning their MBSR course, and MT group participants were scanned within 4 weeks of course completion.
Experiment training procedure
Participants were trained on three experimental tasks, breath monitoring (‘Breathe’), cognitive suppression (‘Suppress’) and working memory maintenance (‘Maintain’) prior to fMRI data acquisition. For the Breathe task, participants were asked to attend to all sensory aspects of their breath (i.e. in the nose, throat, chest and diaphragm), without intentionally altering their respiratory rhythm and with their eyes open. In the event of mind-wandering, participants were asked to calmly return their attention to the breath. For the Suppress task, participants were asked to read foveally presented words while inhibiting any cognitive or emotional response, keeping their minds blank while attending to the word stimulus. For the Maintain task, participants were asked to press a key whenever a word was repeated in a visually presented sequence (a ‘1-back’ task).
Experimental task
The block design experiment was composed of randomized, alternating blocks of IA (Breathe) and EA (Maintain and Suppress) tasks. Each block was 36 s in duration preceded by a 10 s instruction screen consisting of a cue picture and the task name. One run in the scanner consisted of two repetitions of each condition and each participant completed two runs. Task order was fully counterbalanced across participants.
In the IA (Breathe) task, a fixation cross appeared in the centre of the projection screen for the duration of the task block. Participants were instructed to monitor the fixation cross with their eyes open while attending to respiratory sensation, in order to limit confounds that would be introduced by having eyes open in EA and closed in IA.
In the EA tasks (Maintain and Suppress), a word appeared on the screen every 6 s for 4 s followed by a 2 s blank screen, approximating the average respiration rate (Sherwood, 2006) to match the durations of word and breath stimuli. In the Maintain task, only one word in each block was repeated, in a randomized position in the word list. To approximate the self-focus demands of the breath monitoring task, trait adjectives were chosen as word stimuli for the EA tasks, constructed from a well-established list of personality-trait words (Anderson, 1968) and randomly assigned to condition.
Imaging setup
Imaging data was collected with a Signa 3-T MRI system (CV/i hardware, LX8.3 software; General Electric Medical Systems, Waukesha, Wis.) with a standard quadrate birdcage head coil. Stimulus presentation was controlled by the Presentation software package (version 9.81; Neurobehavioral Systems, Inc., Albany, Calif.). Stimuli were presented on a rear-mounted projection screen, set at a (native) 1024 × 768 resolution.
Structural imaging
For each participant, a three-dimensional magnetization-prepared rapid acquisition gradient echo pulse sequence was used to obtain a high-resolution T1-weighted structural volume. The imaging parameters were as follows: repetition time (TR) = 2000 ms; echo time (TE) = 2.63 ms; matrix = 256 × 192; FOV = 256 × 256; slice thickness = 1.3 mm thick; 192 oblique axial slices; total acquisition time = 6.5 min.
Functional imaging
Functional MRI (fMRI) was conducted using T2*-weighted single-shot spiral in-out k-space trajectories optimized for sensitivity to the blood-oxygenation-level-dependent (BOLD) effect [TE/TR/flip angle = 30 ms/2000 ms/70°, 20 cm field-of-view (FOV), 5 mm slice thickness, 64 × 64 matrix, 26 slices in oblique axial orientation].
Data analysis
Interoceptive sensitivity pilot study
Pilot analyses were conducted to ensure interoceptive sensitivity to the respiratory signal. Seven pilot participants (who did not participate in the study) performed a breath monitoring task in which they monitored their respiration, and pressed a button every 8th breath. Participants performed this task in four 3 min blocks, with accuracy assessed relative to respiration belt signal. Total errors were computed as the deviation from eight breaths at each button press, summed across all monitoring blocks. We observed near-perfect levels of respiration monitoring accuracy across the pilot study participants (mean accuracy = 0.97, s.d. = 0.03). Given our desire to observe IA as directly as possible, we elected not to employ a breath counting task in the current study, instructing participants instead to attend purely to respiratory sensation.
Respiration analysis
While participants were instructed not to modify their respiration during IA, altered awareness of respiration may result in respiratory slowing effects (Jevning et al., 1992), potentially affecting the BOLD response (Birn et al., 2006). To control for respiratory changes between IA and EA conditions, respiration rate, phase and respiratory volume per time (RV/T) for each TR of scanning were derived from participant respiration belt data. Respiratory phase (the cycle of inspiration and expiration) is linked to motion-related noise in fMRI data and was used as a nuisance regressor in the first level of analysis. Average respiratory rate for each task block was also modeled at the second (between subjects) level of analysis to control for its effects on BOLD activity.
Functional preprocessing
Functional activity was assessed from the BOLD signal using Statistical Parametric Mapping (SPM8, University College London, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Following reconstruction (SPM8 DICOM import utility), time series data were spatially re-aligned to correct for head motion during functional scans and co-registered with their T1-weighted structural image. The T1 image was bias corrected and segmented using template (International Consortium for Brain Mapping) tissue probability maps for gray matter, white matter and CSF. Warping parameters were obtained from the segmentation procedure and applied to resample the time series data to 3 mm3 voxels, normalizing the data into a common stereotactic reference space (MNI). Data were spatially smoothed using a 6 mm3 full-width half maximum Gaussian kernel. Finally, global mean detrending was used to control for changes in global BOLD signal across the time series (Macey et al., 2004).
First-level statistical models
Following preprocessing, single subject time series were submitted to a general linear statistical model (Friston et al., 1994). Task-specific boxcar stimulus functions were convolved with the canonical hemodynamic response function, modeling the onsets of the Breathe, Suppress and Maintain tasks, as well as a covariate to account for respiratory phase (detailed above). To clarify the contribution of the task conditions, we also modeled a task-free baseline condition, obtained by averaging together the 10 s cue periods preceding each task block.
Due to technical difficulties, only 7 of the 16 participants in the untrained group had complete respiration data, whereas complete respiration data was obtained from all 20 MT participants. All results presented below were tested with both the full cohort of participants as well as with the reduced sample with complete respiration data. Result values are reported for the full cohort, but only reported where both models achieved statistical significance.
Second-level statistical models: insula anatomical region of interest analysis
Eight gray matter region of interests (ROIs) were selected according to the anatomical divisions of the insular gyri, ranging from the anterior accessory gyrus, through the short and long gyri of the middle insula, and into the short and long gyri of the posterior insula (Craig, 2009). Gyri were defined using the high-resolution T1-weighted anatomical volume to which all functional data was normalized. Since the cellular layer divisions of the insula do not neatly segregate by gyrus, in anterior insula zones, the accessory and short gyri were also partitioned into dorsal and ventral zones to characterize the anterior insula distinction between dysgranular and agranular cellular layers (Chikama et al., 1997), yielding eight anatomically defined regions each for the right and left insula (16 ROIs total). Insular ROIs were hand-painted using the MRIcron software package (http://www.sph.sc.edu/comd/rorden/mricron/). ROIs were exclusively masked to ensure that no overlapping voxels were selected, yielding a minimum of 27 voxels in each volume (Figure 2A). Mean time courses were extracted from each ROI using the MarsBar toolbox for SPM (http://marsbar.sourceforge.net). Percent signal change from each of the insula ROIs were extracted for both the IA and EA conditions. ROIs were statistically analyzed using a 2 (group) × 2 (attention) × 2 (left vs right hemisphere) × 8 (anterior to posterior seed location) mixed-model ANOVA.
Fig. 2.
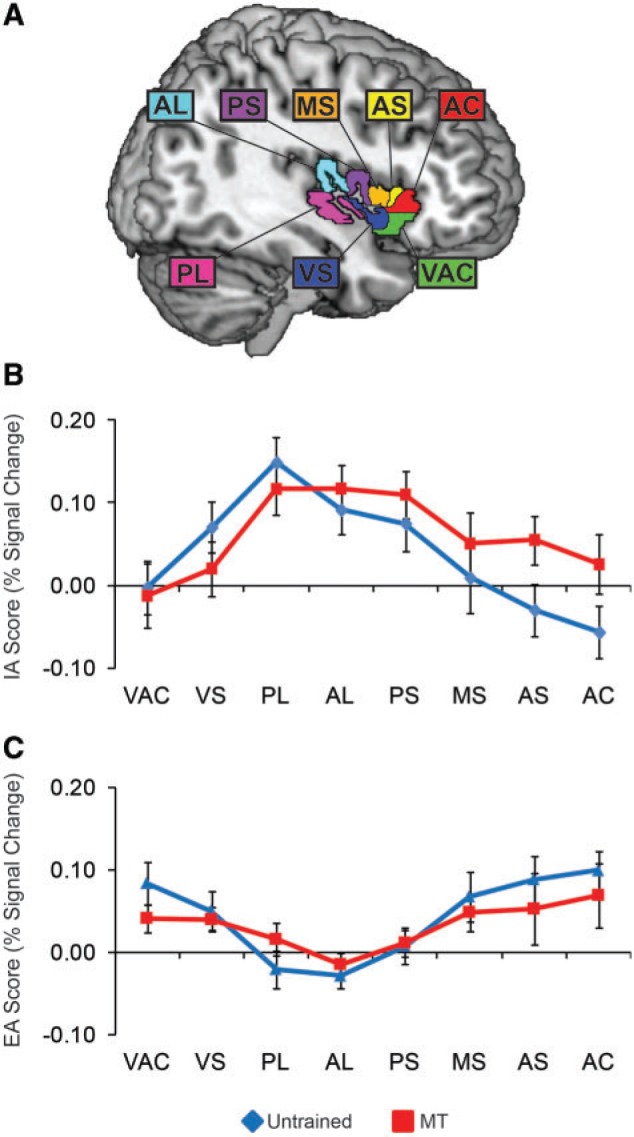
Insula attention activation by anatomical partition. A significant interaction between attention, anatomical partition and MBSR training was found, such that dorsal anterior insula IA activation was greater in the MT than untrained group. (A) A sagittal view of the eight anatomical ROIs drawn to fit each gyrus of the insula on a template brain. (B) Percent signal change plots for IA recruitment as a function of insula partition location. (C) Percent signal change plots for EA recruitment as a function of insula partition location. Error bars represent s.e. VAC, ventral accessory gyrus; VS, ventral short gyrus; PL, posterior long gyrus; AL, anterior long gyrus; PS, posterior short gyrus; MS, middle short gyrus; AS, anterior short gyrus; AC, accessory gyrus.
Second-level statistical models: whole brain analysis
To examine differential effects of attentional focus (IA vs EA) and training (untrained vs MT), contrasts maps for each experimental condition (Breathe, Suppress and Maintain) were analyzed at the second level using a full-factorial mixed-model ANOVA. Within this model, the Breathe condition was contrasted against the Suppress and Maintain conditions to model the effect attention (IA vs EA). To examine training effects on attention, the interaction between attention (IA vs EA) and group (untrained vs MT) was also evaluated. To investigate neural correlates of respiratory activity, mean respiration rate for each task block was also modeled as a regressor in the ANOVA.
All group level t contrasts used a voxel height threshold of Puncorrected < 0.005 (t > 2.61), but at a voxel extent threshold of K ≥ 50, equivalent to a familywise error rate of PFWE < 0.01, as determined in a Monte Carlo simulation of our data (AlphaSim, http://afni.nih.gov/afni/docpdf/AlphaSim.pdf). A more liberal exclusive masking technique was also explored to better characterize the activation related to each task condition, and is elaborated upon in Supplementary Data.
Second-level statistical models: functional ROI analysis
Two ROIs were identified for functional analysis. The first seed region was functionally derived as the sole region demonstrating an attention × group interaction in the whole-brain functional analysis described earlier. The second seed region was selected using an a priori posterior insula region, located at co-ordinates [x = 39; y = −21; z = 21]. Referred to as the ‘interoceptive seed’ in the present study, we observed in prior work (Farb et al., 2012) that this posterior insula region was uniquely associated with variability in respiratory rate during IA, and this association was significantly stronger during IA than during EA. For both the of the seed regions, a 3 mm radius spherical seed ROI was created at the peak voxel location.
To investigate the significance of the ROIs, we employed separate psychophysiological interaction (PPI) analyses using each of the ROIs as seed regions. The PPI analysis modeled the effects of attention condition, activity predicted by the seed region and the interaction between attention and seed region activity in a single ANOVA (Friston et al., 1997).
Finally, we assessed connectivity between the functional seeds and the insula anatomical partitions. For both the attention and interoceptive seed ROIs, main effects of connectivity and PPI scores were extracted from each participant’s PPI analysis map using each of the anatomical insula ROIs. Three-way mixed model ANOVAs employing group (untrained vs MT), hemisphere (right vs left) and anatomical partition as factors assessed whether connectivity between the seed ROIs and the surrounding insula was modulated by MT.
Practice effects
As an additional indicator of how MT modulated recruitment of interoceptive cortices, we examined whether MBSR practice compliance accounted for signal variance during the experimental tasks. Within the MT group, we performed an additional ANOVA using the contrast of IA and EA, including a covariate for the percentage of MBSR daily practice completed. To determine regions of IA or EA-related activity sensitive to practice duration, we performed a conjunction analysis between task-related activity (IA > EA or EA > IA) and the practice covariate. As practice completion was orthogonal to the task contrast, we adopted a more liberal threshold of P < 0.05 (t ≥ 1.73) for each of the conjunction elements, yielding a conjoint probability of P < 0.0025 (t ≥ 3.20), while maintaining the experiment-wide cluster extent threshold of k ≥ 50.
Additionally, we examined the association between practice and anatomical partitions of putative primary interoceptive cortex in the right insula. Aspects of the right insula were directly associated with variations in respiratory rate during IA and were therefore likely candidate regions for practice-related refinement of brain activity during IA relative to EA.
Structural comparisons
To characterize brain structure in the Untrained and MT groups, probability maps were created for each participant using the voxel-based morphometry (VBM8) toolbox in SPM (SPM8; Wellcome Department of Imaging Neuroscience), with default parameters. Images were tissue-classified into gray and white matter, and DARTEL warped into a common space, including both linear and non-linear components in the estimation of the normalization model. Images were then written using only the non-linear components of the model, controlling for global brain size and orientation while displaying local, non-linear differences in gray matter volume. The modulated images were smoothed with a 4 mm full width half maximum (FWHM) Gaussian kernel. We separately compared gray and white matter maps between the untrained and MT groups using between-groups ANOVA models.
RESULTS
Respiration analyses
Mean respiratory signals were analyzed as a function of attention condition, with (i) respiration rate, (ii) volume and (iii) respiration volume/time (RV/T; a measure of respiratory efficiency) analyzed separately in 2 (group) × 2 (attention) mixed model ANOVAs. For respiratory rate, a significant main effect of attention was found [F(1,25) = 9.607, P = 0.005], with slower respiration for interoception than exteroception. However this was due to an interaction between attention and group [F(1,25) = 6.620, P = 0.016] with interoceptive slowing driven by the MT group rather than the untrained group: MT participants respiratory frequency slowed by ∼0.05 Hz or three breaths per minute during interoception, while untrained participants did not slow their breathing (Figure 1A). Respiratory volume showed a complimentary pattern of results, with a main effect of attention demonstrating greater volume during interoception [F(1,25) = 10.265, P = 0.004]. The attention × group interaction for volume was also significant [F(1,25) = 8.330, P = 0.008], with reliably greater volume for the MT interoception condition than the other experimental conditions (Figure 1B).
Fig. 1.
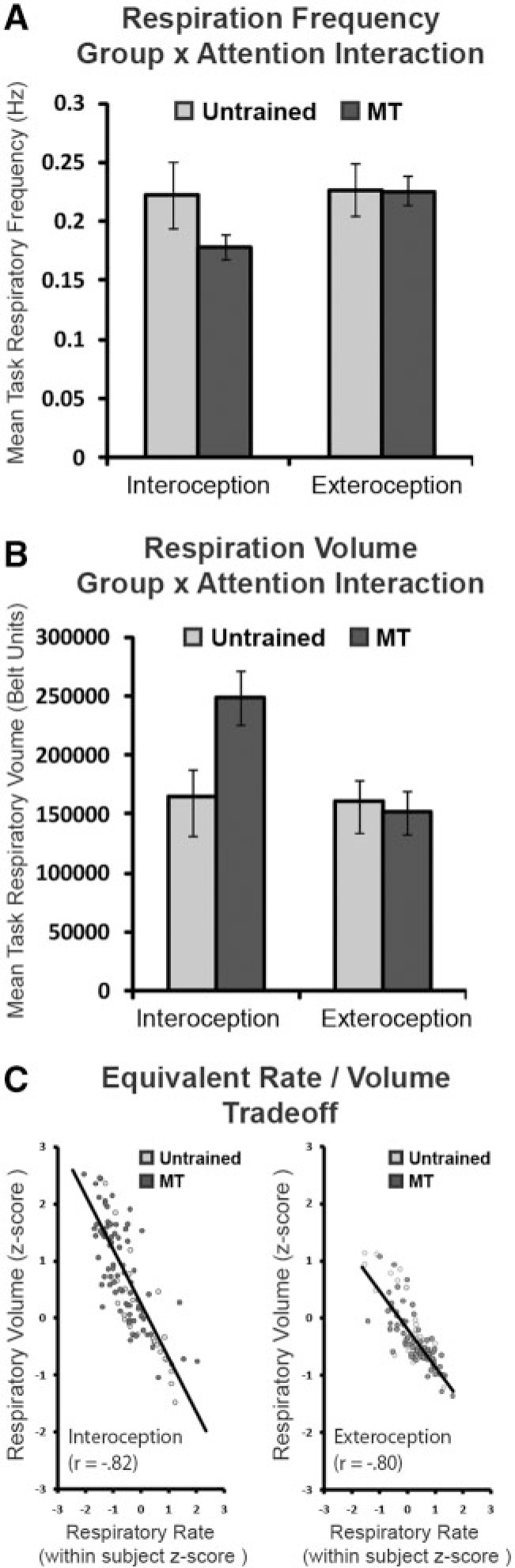
Respiration signal analyses. (A) Mean respiratory frequency (Hz) as a function of attention condition, as derived from a breath counting algorithm. The MT group displayed slower respiration during IA relative to the untrained group, whereas the groups did not differ during EA. (B) Mean respiratory volume (arbitrary respiration belt units) as a function of attention condition, as derived from a breath counting algorithm. The MT group displayed greater respiratory volume (deeper breaths) during IA relative to the untrained group, whereas the groups did not differ during EA. (C) Rate/volume tradeoffs for respiration as a function of attention condition. The slopes did not differ as a function of attention condition or group.
Despite slower and deeper breathing uniquely observed during interoception in the MT group, respiratory efficiency (RV/T) appeared to be equivalent across groups and attention conditions, with no significant main effects or interaction; the relationship between rate and volume was further investigated in a correlation analysis, using rate and volume estimates from each participant task block (Figure 1C). Respiratory rate was strongly negatively correlated with respiratory tidal volume [r(214) = −0.85, P < 0.001], such that faster breaths were shallower; however, this rate/volume relationship was equivalent between interoceptive and exteroceptive tasks [rinteroception = −0.82, rexteroception = −0.80, zfischer’s(106,106) = 0.36, n.s.] and participant groups [runtrained = −0.83, rMT = −0.85, zfischer’s(54,158) = −0.40, n.s.]. Thus any BOLD differences between IA and EA or between untrained and MT participants are unlikely to originate in BOLD confounds related to respiratory efficiency. However, the presence of attention-related respiratory rate and volume changes in the MT but not untrained group emphasizes the importance of controlling for respiration in the examination of MT effects.
Training-related differences in IA
Insula anatomical ROI analysis
We began with a detailed examination of differences in attentional recruitment (IA vs EA) between the two groups in anatomically defined insular subregion ROIs (Figure 2). A three-way interaction was found between ROI location, attention condition and group [F(7,238) = 3.02, P < 0.005]. The interaction appeared to be driven by greater MT group responses to IA in dorsal anterior insula regions, which in the untrained group was more responsive to EA. Post hoc tests confirmed this interpretation, revealing significant group by attention interactions in the accessory gyrus [F(1,34) > 4.50, P = 0.041] and anterior short gyrus [F(1,34) = 7.14, P = 0.012].
Whole brain analysis
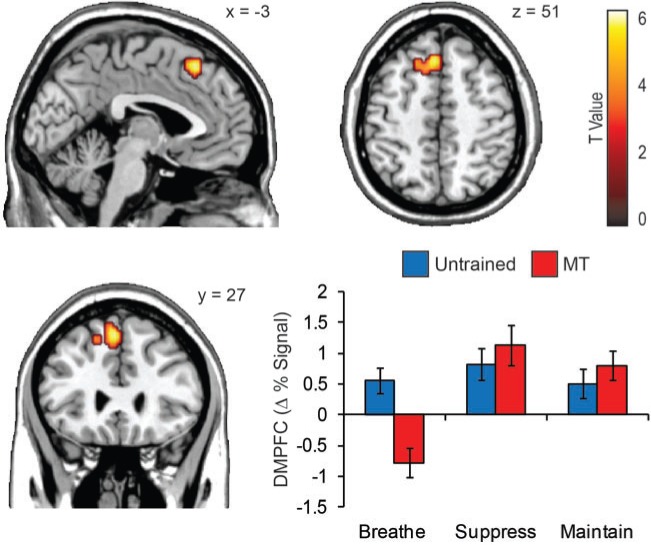
Similar main effects of attention were identified in the control group (Table 1) and MT group (Table 2). The dorsomedial prefrontal cortex (DMPFC; BA 8, at peak P < 1 × 10−5; x = −3, y = 27, z = 51; k = 79) demonstrated a unique interaction between group and attention (Figure 3). Subsequent signal extraction revealed a consistent pattern of DMPFC activation during IA and EA in the untrained group [t(15) = 0.71, n.s.], but reduced activity during IA relative to EA in the MT group [t(19) = 6.82, P < 0.001].
Table 1.
Differences in regional brain activity between interoceptive and exteroceptive attention in controls
| Anatomic region | Cluster | Co-ordinates (mm) |
|||||
|---|---|---|---|---|---|---|---|
| BA | Side | Size | Peak Z | x | y | z | |
| Interoception > exteroception | |||||||
| Paralimbic cluster | 7098 | ||||||
| Retrosplenial cortex | 30 | B | 6.60 | 27 | −51 | 12 | |
| Mid-cingulate | 6/23/24 | B | 5.57 | 15 | −12 | 51 | |
| Parahippocampus | 27 | B | 5.51 | −30 | −42 | −6 | |
| Auditory cortex | 42 | B | 5.45 | 45 | −24 | 24 | |
| Extrastriate | 17 | B | 5.29 | −24 | −51 | 6 | |
| Pulvinar of thalamus | – | B | 5.15 | −9 | −24 | 21 | |
| Insula | 48 | B | 5.05 | −33 | −6 | 15 | |
| Posterior cingulate | 23 | B | 4.59 | −12 | −60 | 15 | |
| Cerebellar vermis | – | B | 302 | 4.54 | −9 | −48 | −45 |
| Exteroception > interoception | |||||||
| Cerebellum/V1/extrastriate | 18/19 | B | 2838 | 6.38 | 3 | −81 | −27 |
| Superior and inferior parietal | 7 | L | 572 | 4.88 | −42 | −54 | 48 |
| DLPFC, operculum, ant. insula and caudate | 44/47/48/25 | L | 566 | 4.86 | −48 | 15 | 21 |
| Superior and inferior parietal | 7 | R | 379 | 4.82 | 45 | −48 | 54 |
| DLPFC, operculum, ant. insula | 44–46 | R | 757 | 4.44 | 36 | 57 | 18 |
| DMPFC and SMA | 8/6 | – | 304 | 4.06 | 0 | 18 | 63 |
| Operculum/ant. insula | 47/48 | R | 176 | 3.69 | 39 | 24 | 0 |
Note: R, right; L, left; B, bilateral; in the case of bilateral activations, the peak listed is for the side with the greater peak activation; V1, primary visual cortex; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; SMA, supplementary motor area.
Table 2.
Differences in regional brain activity between interoceptive and exteroceptive attention in the MT group
| Anatomic region | Cluster | Co-ordinates (mm) |
|||||
|---|---|---|---|---|---|---|---|
| BA | Side | Size | Peak Z | x | y | z | |
| Interoception > exteroception | |||||||
| Paralimbic cluster | 9837 | ||||||
| Insula | 48 | B | 6.81 | 33 | −6 | 15 | |
| Mid-cingulate | 6/23/24 | B | 5.95 | 15 | −21 | 51 | |
| Pulvinar of thalamus | – | B | 5.91 | −21 | −18 | 21 | |
| Posterior cingulate | 23 | B | 5.84 | −12 | −54 | 21 | |
| Retrosplenial cortex | 30 | B | 5.61 | 27 | −51 | 9 | |
| Parahippocampus | 27 | B | 5.57 | 33 | −36 | −3 | |
| Paracentral gyrus | 3–6 | B | 5.54 | −30 | −18 | 36 | |
| Cerebellum (area 8/9) | R | 52 | 3.92 | 18 | −60 | −48 | |
| Brainstem | B | 198 | 3.61 | 0 | −42 | −45 | |
| Exteroception > interoception | |||||||
| DMPFC | 32/8 | B | 1969 | 7.28 | −3 | 27 | 51 |
| Inf. parietal, visual | 39/40/17–18 | L | 5663 | 6.42 | −45 | −54 | 51 |
| DLPFC, operculum, head of caudate | 44–47/6/25 | B | 2283 | 5.75 | −51 | 12 | 30 |
| Inf. parietal, angular | 7/39/40 | R | 866 | 4.92 | 33 | −57 | 54 |
| Inferior temporal pole | 38 | R | 111 | 4.29 | 57 | 0 | −39 |
Note: R, right; L, left; B, bilateral; in the case of bilateral activations, the peak listed is for the side with the greater peak activation.
Fig. 3.
DMPFC activity. The dorsomedial prefrontal cortex (DMPFC) was the only region wherein attention (IA vs EA) condition interacted with group (untrained vs MT). The bar graph displays DMPFC task activations relative to the within-participant baseline period.
Functional connectivity
To index primary interoceptive cortex, we identified a right posterior insula region related to variations in respiratory rate between task blocks. This interoceptive seed region was also strongly responsive to IA over EA in both groups [t(1,34) = 5.76, P < 1 × 10−5] (Figure 4A), serving as a common region wherein attention modulates the representation of interoceptive signal. The interoceptive seed was therefore a reasonable point of origin from which attentional training may affect the cortical propagation of interoceptive signal.
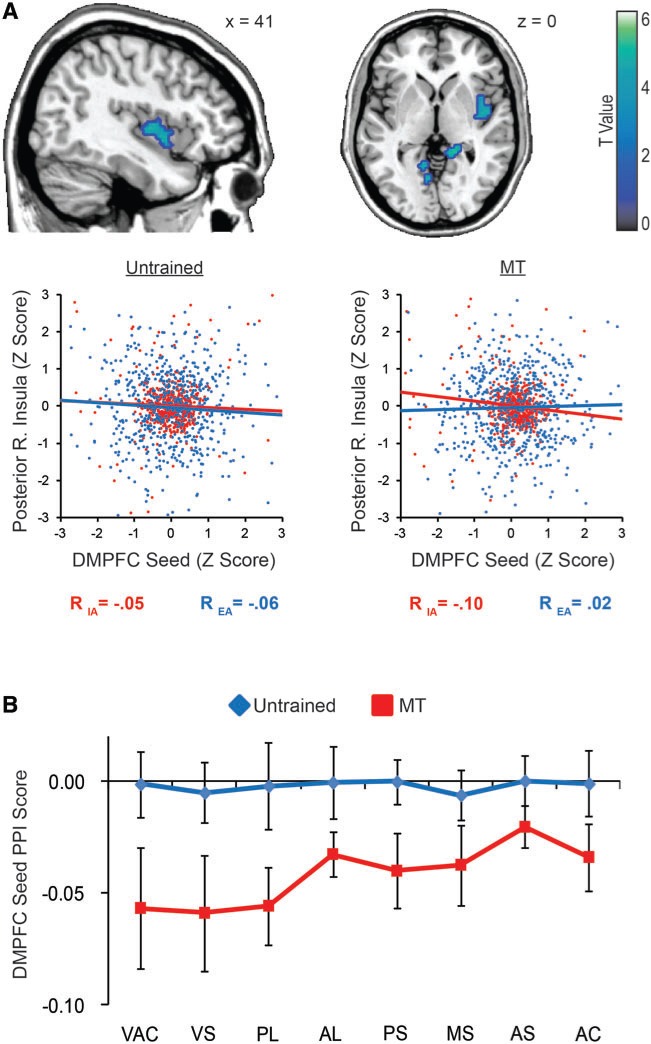
Fig. 4.
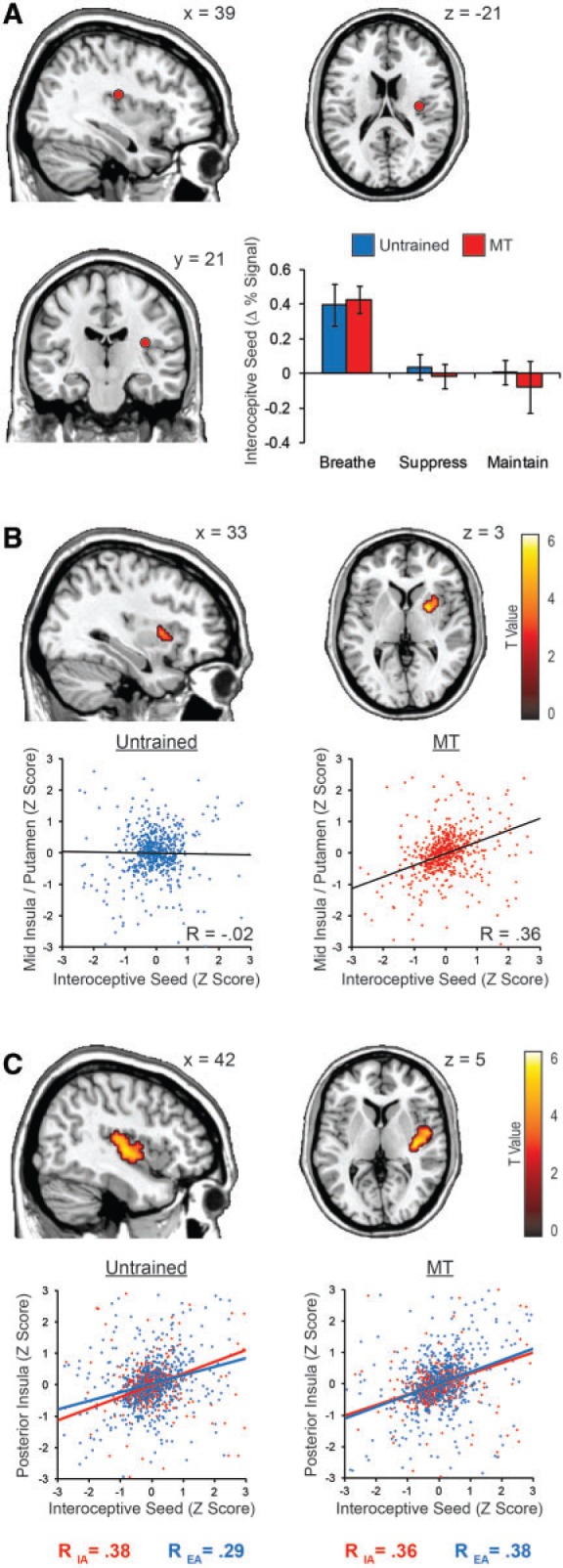
Primary interoceptive seed activity and connectivity. A posterior insula region whose activity was associated with variation in respiratory rate during IA but not EA was selected as a primary interoceptive seed region for further analysis. The 3 mm spherical interoceptive seed region proved to be sensitive to the IA vs EA contrast (A). Comparison of whole brain functional connectivity revealed greater interoceptive seed connectivity with the middle insula/putamen in the MT group relative to the untrained group, irrespective of attention condition (B). Group differences in the PPI analysis suggested that in the untrained group, the posterior and middle insula demonstrated greater seed connectivity during IA than EA, reaching levels of connectivity observed across conditions in the MT group (C). Scatterplot r-values display the Fisher Z transformed mean of the within-subject correlations for each group. The scatterplots themselves show TR by TR activation patterns from a single representative participant in each group, whose within-subject correlation best matched the mean within-subject correlation for the group.
We used the interoceptive seed to examine group differences in condition-independent (main effect of ROI) and dependent (PPI) functional connectivity (Table 3). Independent of attention conditions, MT was associated with higher functional connectivity between the interoceptive seed and the right middle putamen, extending into the short gyrus of the anterior insula [t(1,34) = 4.02, P < 0.001; x = 24, y = 3, z = 3] (Figure 4B). Condition-dependent connectivity was observed across groups, in the cerebellar vermis and in the posterior insula just rostral to the primary interoceptive seed, with higher connectivity in IA relative to EA. However, this posterior insula region also displayed a between-groups PPI difference, revealing that the condition-dependent insula connectivity was driven by the untrained group (Figure 4C). Subsequent analyses of the PPI correlation coefficients suggested that the untrained group elevated insula connectivity during IA to match the condition-independent connectivity levels observed within the MT group.
Table 3.
Summary of psychophysiological interactions (PPI) between attention condition (IA vs EA) and seed region
| Anatomic region | Cluster | Co-ordinates (mm) |
|||||
|---|---|---|---|---|---|---|---|
| BA | Side | Size | Peak Z | x | y | z | |
| Interoceptive seed: main effects | |||||||
| Cerebellum (vermis) | – | – | 81 | 3.80 | 0 | −72 | −33 |
| Posterior insula | – | R | 77 | 3.18 | 51 | −9 | 3 |
| Interoceptive seed: untrained > MT | |||||||
| Posterior insula | – | R | 223 | 3.70 | 51 | −9 | 0 |
| DMPFC seed: main effects | |||||||
| Calcarine/lingual gyrus | 17 | L | 171 | −3.46 | −21 | −72 | 18 |
| Parahippocampus | 27 | R | 64 | −3.44 | 18 | −48 | 6 |
| Posterior insula | – | R | 104 | −3.10 | 39 | −9 | 3 |
| DMPFC seed: MT > untrained | |||||||
| Posterior insula | – | L | 56 | 4.70 | −48 | 0 | 9 |
Note: R, right; L, left. DMPFC, dorsomedial prefrontal cortex, the attention × group seed region from the whole brain ANOVA. Positive z values represent positive PPI terms, suggesting more positive correlation during IA than EA, whereas negative z values represent negative PPI terms, suggesting more negative correlation during IA than EA.
We next performed a connectivity analysis to explore the functional role of the DMPFC region identified in the whole brain analysis. Condition-dependent connectivity was observed across groups (Table 3): more negative DMPFC connectivity was observed during IA than EA with the right posterior to middle insula, right parahippocampal gyrus and left calcarine gyrus. However, the right insula effect appeared to be driven by the MT group, which had a significantly greater PPI effect [t(1,34) = 3.45, P < 0.005] (Figure 5A). A between-group PPI difference was also observed between the DMPFC and the left posterior insula, such that IA promoted negative connectivity with the left insula in the MT group but not the untrained group. Furthermore, an analysis of connectivity within the anatomical insula ROIs revealed a main effect of group across the insula [F(1,34) = 4.27, P < 0.05], such that only the MT group demonstrated a negative PPI effect within the insula ROIs (Figure 5B).
Fig. 5.
DMPFC seed connectivity. The DMPFC was used as a seed ROI in a psychophysiological interaction (PPI) analysis, and these PPI maps were compared between untrained and MT groups. IA relative to EA predicted negative insula connectivity, as a main effect in the right posterior insula, an effect that appeared to be driven by the MT group in both whole brain (A) and insula ROI analyses (B). Scatterplot r-values display the Fisher Z transformed mean of the within-subject correlations for each group. The scatterplots themselves show TR by TR activation patterns from a single representative participant in each group, whose within-subject correlation best matched the mean within-subject correlation for the group.
Practice effects
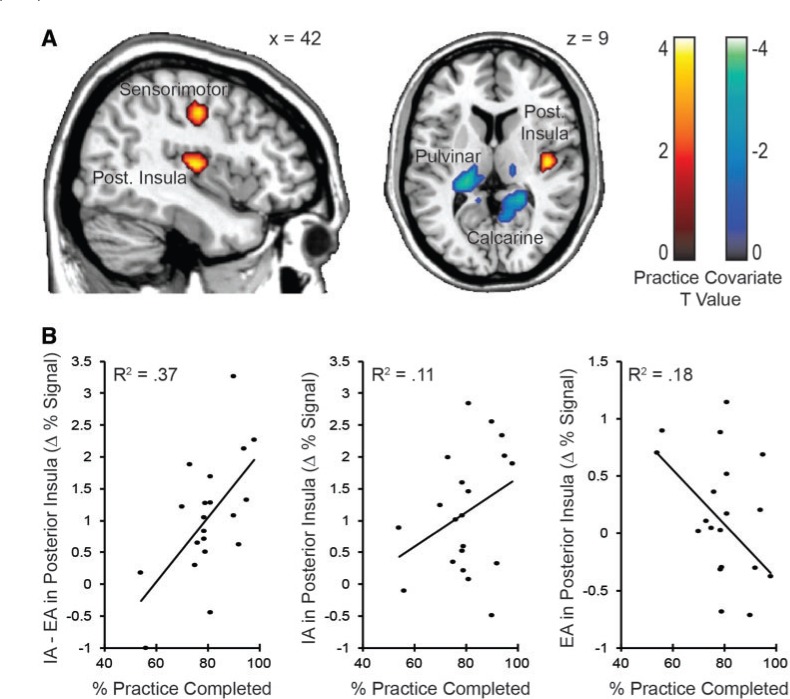
Participants reported good compliance with the daily practice schedule prescribed during MBSR (on an average 78.6 ± 13.4% of practice hours were completed, with a minimum of 54% completion). Across the whole brain, practice compliance was associated with greater IA-related activation in the right sensorimotor cortex and posterior insula, consistent with enhanced attention to primary interoceptive cortex and reduced activation in the left pulvinar and lateral geniculate nuclei of the thalamus and right calcarine gyrus, consistent with reduced attention to primary visual cortex and its subcortical afferents (Figure 6A).
Fig. 6.
Relationship between MBSR daily practice completion and attention-related brain activity. Within the MT group, percentage of practice completion for each participant was entered as a covariate into the IA vs EA design matrix. The brain maps display significant conjunction regions between attention-related activity and the practice completion related activity. (A) Areas of IA > EA activation that are related to practice completion. (B) Correlations between activity in the right insula anterior long gyrus anatomical ROI and practice completion. Variance accounted for by practice completion (R2) is displayed for attentional bias (IA–EA), as well as the simple effects of IA and EA separately.
We further examined the specificity of practice effects in the eight anatomical right insula partitions. After correcting for multiple comparisons, we found that bias toward interoceptive activation (IA–EA activation) in the posterior long gyrus of the right insula was correlated with percentage of practice completed [rIA–EA(18) = 0.61, pcorrected < 0.05] (Figure 6B). This effect appeared to be driven both by reduced activity during EA with higher practice levels [rEA(18) = −0.42, P = 0.06] and greater activity during IA with higher practice levels [rIA(18) = 0.33, P = 0.16].
Structural analyses
To determine whether IA training was associated with structural differences, we compared whole-brain gray matter and white matter volumes between the untrained and MT groups. We observed greater gray matter volume in the MT group in the left caudate nucleus (at peak P < 1 × 10−4, x = −15; y = 8; z = 22, cluster size = 362). Practice did not reliably predict gray matter change [r(18) = 0.28, n.s.]. No training-related differences in gray matter volume were observed in the anatomical insula ROIs or the functional seed ROIs.
DISCUSSION
The present study investigated whether IA practice through MT resulted in functional plasticity in interoceptive representation cortex. Relative to untrained, waitlisted control participants, individuals completing an 8 week MBSR course demonstrated IA-specific functional plasticity in the middle and anterior insula, regions theorized to support present moment awareness (Farb et al., 2007; Craig, 2009), commensurate with the goals of the mindfulness intervention. We were also able to demonstrate two novel mechanisms by which MT may modulate the neural propagation of interoceptive signal from the posterior insula during IA: (i) MT may promote greater functional connectivity between the posterior insula and anterior insula gyri, leading to greater anterior insula activation and (ii) MT may simultaneously reduce DMPFC recruitment and strengthen negative DMPFC/insular connectivity. Supporting the idea that it was specifically the interoceptive practices that drove these group differences, practice compliance predicted greater IA-selective recruitment of the posterior insula, putative primary interoceptive cortex (Craig, 2002; Farb et al., 2012). Thus, the present work suggests an emergent prefrontal pathway through which MT alters IA.
Prefrontal involvement in interoception
In whole brain analyses, activity in the DMPFC was uniquely implicated in the interaction between attentional focus (IA vs EA) and experimental group (untrained vs MT), demonstrating reduced IA-related activity in the MT but not untrained group. DMPFC deactivation has been documented in exogenous stimulation of interoceptive pathways such as gastric distention (van Oudenhove et al., 2009), and more generally such deactivation is consistent with disengagement from ‘default mode’ processing in cortical midline structures, typically observed during tasks requiring cognitive control (Northoff and Bermpohl, 2004; Seeley et al., 2007). Conversely, DMPFC activation has been related to deployment of focal attention, acting as an index of executive processes that are present both during effortful task-related concentration (Seeley et al., 2007; Mullette-Gillman and Huettel, 2009), and during unintentional mind-wandering (Christoff et al., 2009). DMPFC deactivation during IA therefore suggests a functional departure from both mind-wandering and focal attention states, comparable to effects observed during exogenous interoceptive cuing. Since MT practice compliance correlated with insular rather than DMPFC activity, DMPFC deactivation may not indicate a change related to expertise in attentional focus, but rather signal a qualitative shift in attentional stance. This hypothesis is consistent with previously reported effects of MT, shifting resources from evaluative, cortical midline processing to an expansive and diffuse form of sensory attention such as the interoceptive insula representation shown here (Farb et al., 2007, 2010).
Additional insights into the functional implications of DMPFC deactivation are available from our connectivity analyses. The DMPFC demonstrated training-related plasticity consistent with the development of an IA network: following MT, the DMPFC demonstrated IA-specific negative connectivity to primary interoceptive cortex in the posterior insula. Combined with DMPFC deactivation during IA, this negative connectivity could serve to sustain positive activation in interoceptive representation areas, although the causal direction of this connectivity pattern is unknown. Regardless of network directionality, the IA-specific connectivity pattern limits unintentional interoceptive suppression when the DMPFC is activated for executive functions, as DMPFC-insula connectivity was absent during EA. So, while MT is associated with the skillful reduction of DMPFC during IA that promotes interoceptive recruitment, this relationship is context-specific. During periods of prefrontal activation such as EA, condition-dependent connectivity allows for interoceptive tone to be preserved rather than automatically suppressed by DMPFC activation, whereas such suppression has been evident following emotional challenge in untrained participants (Farb et al., 2010). Allowing for the preservation of interoceptive tone in EA is an important facet of the MBSR program, which aims to bring the expansive quality of IA to interactions with the external world.
The present findings suggest an important role by which the DMPFC facilitates MT effects, promoting reduced conceptual cortical activity and enhanced interoceptive connectivity. DMPFC deactivation could therefore be one neural mechanism of attentional control enhancing interoceptive representation following MT.
Enhanced propagation of interoceptive signal
In addition to altered cortical midline prefrontal-insula connectivity, training related plasticity was observed within the insula itself, between primary interoceptive cortex in the posterior insula and adjacent short gyri of the middle insula. Untrained participants demonstrated task-dependent connectivity between the posterior region and more anterior insula zones, selectively increasing insula connectivity during IA relative to EA. In contrast, the MT group demonstrated task-independent connectivity between the posterior and middle insula, matching the untrained group’s level of IA-specific intra-insula connectivity. Thus, while untrained participants were able to voluntarily invoke IA to promote connectivity of interoceptive signal from the primary interoceptive cortex toward more anterior sensory integration regions, MT participants appeared to posses this increased connectivity by default, regardless of task-demands. Such a finding is consistent with a second goal of MBSR practices, to provide individuals with a consistent ‘online’ representation of body awareness even in the face of exogenously cued stressors, weakening enduring conceptual evaluations with competing knowledge of constantly changing interoceptive sensations (Kabat-Zinn, 1990). To formally test this hypothesis, future research could assess whether levels of baseline intra-insula connectivity predict the frequency of spontaneous experiences of interoceptive self-reference in the absence of experimental task demands.
In addition to the connectivity findings discussed earlier, MT was also associated with modulation of interoceptive signal amplitude. While the primary interoceptive region in the posterior insula did not demonstrate increased IA-related recruitment in the MT group, MBSR practice compliance within the MT group was associated with increased attentional modulation of posterior insular cortex, consistent with experience dependent modulation of primary interoceptive representations. Additionally, analogous to expanded representation in auditory cortex following learned salience for auditory cues (Polley et al., 2004, 2006), MT enhanced interoceptive representation in adjacent anterior insular cortex, a region more responsive toward exteroceptive than interoceptive signals prior to training. Thus, participating in the MBSR program appeared to facilitate interoceptive integration across the MT group regardless of practice compliance, consistent with an intention to integrate interoceptive information into present moment context. However, only through daily practice was the tone of primary insular interoceptive representation enhanced. In summary, interoceptive intent was only sufficient to increase the extent of interoceptive integration in terms of functional connectivity; MBSR training was required to increase amplitude of signal in these anterior insula integration regions, and daily practice in addition to training was required to also maximize signal amplitude in primary interoceptive representation regions.
While across participants MT effects were observed in more anterior than posterior insula regions, it should also be noted that these effects were observed in dorsal rather than ventral anatomical zones. This distinction is consistent with our understanding of anatomical divisions in the anterior insula: the anterior insula has been functionally divided into a dorsal ‘sensorimotor’ region and a ventral ‘limbic’ region based on cortical projection analysis (Chikama et al., 1997). The dorsal dysgranular layer of the insula includes dense interconnections with supplementary motor and sensorimotor association cortices, whereas the ventral ‘limbic’ region is densely connected with orbitofrontal cortex, amygdala and entorhinal cortex (Mesulam and Mufson, 1982; Carmichael and Price, 1995). Such a distinction fits with the intention of MT to develop interoceptive but non-evaluative awareness, recruiting dorsal viscerosomatic insular cortex distinct from the valence-laden orbitofrontal connectivity of the ventral insula.
Structural findings
Structural analyses suggested that MBSR-related changes in functional activity were not due to modulation of gray or white matter volume. Instead, training was related to greater gray matter volume in the left caudate nucleus. Training-related changes to left caudate anatomy are consistent with altered habitual direction of attention, as the caudate nucleus is important for the habitual direction of attention and behavior (Baxter et al., 1992; Packard and Knowlton, 2002; McNab and Klingberg, 2008). It should be noted that more pervasive gray matter changes were observed in a longitudinal study of MT (Holzel et al., 2011), whereas the current study’s cross-sectional design would be less sensitive to training-related changes due to natural heterogeneity between the different participants in each group, a limitation of the current study’s design. Additionally, it should be noted that because the basal ganglia lie adjacent to the lateral ventricles, they are particularly susceptible to false positives during morphometric analysis (Mechelli et al., 2005). However, because the presently discussed region was identified using a familywise-corrected cluster size threshold of over 250 voxels, many of which extended below the ventricular surface into the deeper nuclei of the caudate, there is good reason to believe that this anatomical finding reflects a real difference in anatomy between groups.
Limitations and future directions
While the present study provides some initial indications of a cortical plasticity related to interoceptive training, it does possess several limitations that should be noted. First, the study employed a cross-sectional design, testing both groups at a single time point. However, because our study uses a cross-sectional design, we were able to compare both groups at their initial exposure to the imaging paradigm, which we believe to be superior to an uncontrolled pre–post longitudinal study in which repetition effects would confound every apparent effect of training. Ideally however, a longitudinal design measuring participants before and after training that also included a control group measured at both time points would provide a higher quality of evidence.
The present work suggests that training-related interoceptive plasticity is possible, but raises many questions as to the impact of interoceptive plasticity on perception and behavior. In future research, it will be important to determine whether training-related increases in insula signal propagation are correlated with measurable improvements in interoceptive acuity. These improvements may in turn explain more general improvements in participant well-being as participants improve their ability to separate interoceptive sensation from emotional appraisal. These considerations notwithstanding, the present study makes important steps toward identifying a candidate mechanism by which MBSR practices produce observable changes to attentional systems (e.g., Jha et al., 2007; Chambers et al., 2008). Through training in IA, increased activity and altered connectivity with regions of the primary and secondary interoceptive cortex, may lead to a richer context for awareness of present moment sensations from the body.
Concluding remarks
We examined whether interoceptive cortical representations demonstrate functional plasticity following 8 weeks of interoceptive monitoring practice. Secondary representations of interoceptive information demonstrated enhanced activity during IA, and greater homework compliance was associated with greater selectivity of primary cortex for interoceptive signals. Rather than simply amplifying the gain of primary interoceptive signals, the interoceptive practices prescribed by MBSR may enhance these signals’ cortical propagation during attention toward distinct sensory features of the breath (David et al., 2008; Ling et al., 2009). Such enhancement may allow attention to more readily select features of the interoceptive signal, integrating them into a broader contextual representation of present-moment sensation.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
FUNDING
Canadian Institute of Health Research (#MT81164; www.cihr-irsc.gc.ca), National Institute of Mental Health (#MH066992; www.nimh.nih.gov), the Women of Baycrest (Fellowship; womenofbaycrest.com) and the Mind and Life Institute (Varela grant; www.mindandlife.org).
Supplementary Material
REFERENCES
- Anderson NH. Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology. 1968;9:272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Barnes SJ, Finnerty GT. Sensory experience and cortical rewiring. Neuroscientist. 2010;16:186–98. doi: 10.1177/1073858409343961. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Schwartz JM, Bergman KS, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Archives of General Psychiatry. 1992;49:681–9. doi: 10.1001/archpsyc.1992.01820090009002. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–48. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Brener J, Jones JM. Interoceptive discrimination in intact humans: detection of cardiac activity. Physiology & Behavior. 1974;13:763–7. doi: 10.1016/0031-9384(74)90259-5. [DOI] [PubMed] [Google Scholar]
- Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for salutary effects. Psychological Inquiry. 2007;18:211–37. [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–86. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–41. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmody J, Baer RA, Lykins LBE, Olendzki N. An empirical study of the mechanisms of mindfulness in a mindfulness-based stress reduction program. Journal of Clinical Psychology. 2009;65:613–26. doi: 10.1002/jclp.20579. [DOI] [PubMed] [Google Scholar]
- Chambers R, Chuen Yee Lo B, Allen NB. The impact of intensive mindfulness training on attentional control, cognitive style, and affect. Cognitive Therapy and Research. 2008;32:302–22. [Google Scholar]
- Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. Journal of Neuroscience. 1997;17:9686–705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8719–24. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proceedings of the National Academy of Sciences of the United States of America. 1998;95:831–8. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews. Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences. 2009;364:1933–42. doi: 10.1098/rstb.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- David SV, Hayden BY, Mazer JA, Gallant JL. Attention to Stimulus Features Shifts Spectral Tuning of V4 Neurons during Natural Vision. Neuron. 2008;59:509–521. doi: 10.1016/j.neuron.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one’s emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10:25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Anderson AK. Attentional modulation of primary interoceptive and exteroceptive cortices. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhr385. Advance Access published January 19, 2012, doi: 10.1093/cercor/bhr385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive & Affective Neuroscience. 2007;2:313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurion A, Cerf B, Le Bihan D, Pillias AM. fMRI study of taste cortical areas in humans. Annals of the New York Academy of Sciences. 1998;855:535–45. doi: 10.1111/j.1749-6632.1998.tb10623.x. [DOI] [PubMed] [Google Scholar]
- Flynn FG. Anatomy of the insula functional and clinical correlates. Aphasiology. 1999;13:55–78. [Google Scholar]
- Fresco DM, Segal ZV, Buis T, Kennedy S. Relationship of posttreatment decentering and cognitive reactivity to relapse in major depression. Journal of Consulting and Clinical Psychology. 2007;75:447–55. doi: 10.1037/0022-006X.75.3.447. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Evans EM, Maraj N, Dozois DJ, Partridge KP. Lettting go: mindfulness and negative auotmatic thinking. Cognitive Therapy and Research. 2008;32:758–74. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Firth CD, Frackowiak RSJ. Statistical Parametric Maps in Functional Imaging: A General Linear Approach. Human Brain Mapping. 1994;2:189–210. [Google Scholar]
- Garland EL, Gaylord SA, Fredrickson BL. Positive reappraisal mediates the stress-reductive effects of mindfulness: an upward spiral process. Mindfulness. 2011;2:59–67. [Google Scholar]
- Gibson EJ. Improvement in perceptual judgments as a function of controlled practice or training. Psychological Bulletin. 1953;50:401–31. doi: 10.1037/h0055517. [DOI] [PubMed] [Google Scholar]
- Gibson EJ. Exploratory behavior in the development of perceiving, acting and the acquiring of knowledge. Annual Review of Psychology. 1988;39:1–41. [Google Scholar]
- Godde B, Erhardt J, Braun C. Behavioral significance of input-dependent plasticity of human somatosensory cortex. Neuroreport. 2003;14:543–6. doi: 10.1097/00001756-200303240-00002. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:10829–37. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RH, Pascual-Leone A. Cortical plasticity associated with Braille learning. Trends in Cognitive Sciences. 1998;2:168–74. doi: 10.1016/s1364-6613(98)01172-3. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–18. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Research. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Gard T, et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive & Affective Neuroscience. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurliman E, Nagode JC, Pardo JV. Double dissociation of exteroceptive and interoceptive feedback systems in the orbital and ventromedial prefrontal cortex of humans. Journal of Neuroscience. 2005;25:4641–8. doi: 10.1523/JNEUROSCI.2563-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovski B, Malhi GS. The psychological and neurophysiological concomitants of mindfulness forms of meditation. Acta Neuropsychiatrica. 2007;19:76–91. doi: 10.1111/j.1601-5215.2007.00175.x. [DOI] [PubMed] [Google Scholar]
- Jancke L, Gaab N, Wustenberg T, Scheich H, Heinze HJ. Short-term functional plasticity in the human auditory cortex: an fMRI study. Brain Research. Cognitive Brain Research. 2001;12:479–85. doi: 10.1016/s0926-6410(01)00092-1. [DOI] [PubMed] [Google Scholar]
- Jevning R, Wallace RK, Beidebach M. The physiology of meditation: a review. A wakeful hypometabolic integrated response. Neuroscience and Biobehavioral Reviews. 1992;16:415–24. doi: 10.1016/s0149-7634(05)80210-6. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive, Affective & Behavioral Neuroscience. 2007;7:109–19. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Joorman J, Siemer M. Affective processing and emotion regulation in dysphoria and depression: cognitive biases and deficits in cognitive control. Social and Personality Psychology Compass. 2011;5:13–28. [Google Scholar]
- Kaas JH. The organization of cortex in mammals: implications for theories of brain function. Annual Review of Psychology. 1987;38:124–51. doi: 10.1146/annurev.ps.38.020187.001021. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. General Hospital Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain and Illness. New York: Delacorte; 1990. [Google Scholar]
- Kourtzi Z, Betts LR, Sarkheil P, Welchman AE. Distributed neural plasticity for shape learning in the human visual cortex. PLoS Biology. 2005;3:e204. doi: 10.1371/journal.pbio.0030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–7. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S, Liu T, Carrasco M. How spatial and feature-based attention affect the gain and tuning of population responses. Vision Research. 2009;49:1194–1204. doi: 10.1016/j.visres.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Brannan S, Egan G, et al. Brain responses associated with consciousness of breathlessness (air hunger) Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2035–40. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM. A method for removal of global effects from fMRI time series. Neuroimage. 2004;22:360–6. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- Maslow AH. A theory of human motivation. Psychological Review. 1943;50:370–96. [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11:103–7. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Current Medical Imaging Reviews. 2005;1:105–13. [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. Journal of Comparative Neurology. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Moberly NJ, Watkins ER. Negative affect and ruminative self-focus during everyday goal pursuit. Cognition & Emotion. 2010;24:729–39. doi: 10.1080/02699930802696849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullette-Gillman OA, Huettel SA. Neural substrates of contingency learning and executive control: dissociating physical, valuative, and behavioral changes. Frontiers in Human Neuroscience. 2009;3:23. doi: 10.3389/neuro.09.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, et al. Functional organization of the human anterior insular cortex. Neuroscience Letters. 2009;457:66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in Neuroscience. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annual Review of Neuroscience. 2002;25:563–93. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Polley DB, Heiser MA, Blake DT, Schreiner CE, Merzenich MM. Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16351–6. doi: 10.1073/pnas.0407586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. Journal of Neuroscience. 2006;26:4970–82. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist. 2000;55:68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Nanez JE, Watanabe T. Advances in visual perceptual learning and plasticity. Nature Reviews. Neuroscience. 2010;11:53–60. doi: 10.1038/nrn2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood L. Fundamentals of Physiology: A Human Perspective. Toronto: Thomson; 2006. [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13:334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- van Oudenhove L, Vandenberghe J, Dupont P, et al. Cortical deactivations during gastric fundus distension in health: visceral pain-specific response or attenuation of ‘default mode’ brain function? A H2 15O-PET study. Neurogastroenterology and Motility. 2009;21:259–71. doi: 10.1111/j.1365-2982.2008.01196.x. [DOI] [PubMed] [Google Scholar]
- Vanni S, Revonsuo A, Saarinen J, Hari R. Visual awareness of objects correlates with activity of right occipital cortex. Neuroreport. 1996;8:183–6. doi: 10.1097/00001756-199612200-00037. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y, Watanabe T. Defining a link between perceptual learning and attention. PLoS Biology. 2008;6:e221. doi: 10.1371/journal.pbio.0060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.