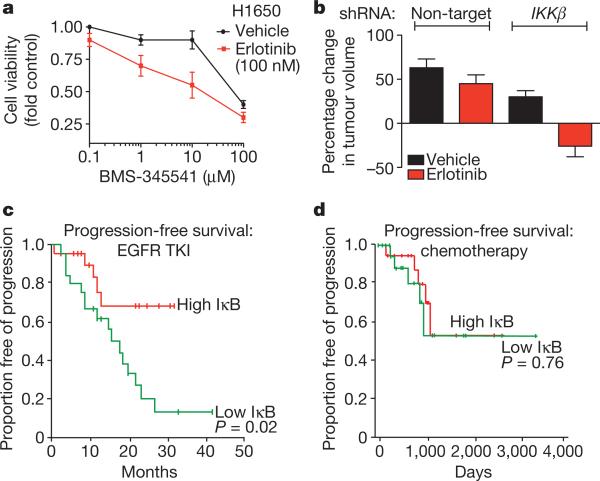
Figure 4. Rationale for combined NF-κB and EGFR inhibition in EGFR-mutant lung cancers.
a, Dose response in H1650 cells treated with BMS-345541 (IKK inhibitor) and additionally either vehicle or erlotinib (100 nM). Viability was measured as in Fig. 1 (n = 3, mean + s.e.m.). b, Effects of stable knockdown of IKKβ on erlotinib sensitivity in H1650 tumour xenografts compared to non-target shRNA control H1650 tumours. Established tumours (>200 mm3, n = 10 per treatment group) were randomized and treated for 7 days with 12.5 mg erlotinib per kg per day or vehicle. Data are expressed as in Fig. 2c (+ s.e.m.). c, d, Effects of IκkB expression on progression free survival in patients with EGFR-mutant lung cancers (c) treated with single agent EGFR TKI (n = 52) or (d) chemotherapy and surgery (n = 43). Clinical characteristics and responses were defined previously22. Median progression-free survival and overall survival for the entire EGFR TKI-treated cohort were 20 months (95% confidence interval, 13–26.9) and 33 months (95% confidence interval, 22.2–43.8), respectively.