Abstract
We validated a handheld point-of-care lactate (POCL) monitor's ability to measure lactate in cerebrospinal fluid (CSF) and diagnose bacterial meningitis in Uganda. There was a strong linear correspondence between POCL and standard laboratory lactate test results (R2 = 0.86; P < 0.001). For 145 patients with clinical meningitis, the area under the receiver operating characteristic curve for the prediction of bacterial meningitis by CSF POCL was 0.92 (95% confidence interval = 0.85–0.99, P < 0.001). A CSF POCL concentration of 7.7 mmol/L provided 88% sensitivity and 90% specificity for the diagnosis of bacterial meningitis. CSF POCL testing had excellent use in the diagnosis of bacterial meningitis, and it may be useful where CSF analyses are delayed or laboratory infrastructure is limited.
Introduction
Bacterial meningitis is a medical emergency that requires early diagnosis and treatment to reduce morbidity and mortality. The diagnosis can be difficult to make because of low sensitivity and specificity of clinical findings, prior antibiotic use, underlying immunosuppression, prior neurosurgery, and in some regions, resource limitations.1–4 Furthermore, the diagnosis requires interpretation of cerebrospinal fluid (CSF) laboratory evaluations. However, CSF lactate concentration can help discriminate between bacterial and non-bacterial meningitis.5–7 Such testing is limited by the requirements of specialized equipment and the maintenance of a cold chain. Reducing laboratory requirements and improving the time to CSF lactate results may improve the ability to diagnose and treat bacterial meningitis. Therefore, we performed a prospective study of the ability of a handheld point-of-care lactate (POCL) monitor to accurately measure CSF lactate and diagnose bacterial meningitis in a regional referral hospital in Uganda.
Materials and Methods
Validation of CSF POCL testing.
We used a handheld POCL monitor (Accutrend Portable Lactate Analyzer; Sports Resource Group Inc., Minneapolis, MN) to prospectively evaluate in duplicate 100 consecutive CSF samples submitted to the clinical laboratory at the University of Virginia for standard laboratory lactate (SLL) testing (Architect; Abbott Laboratories). POCL testing required one drop of CSF, and results were available in 60 seconds.
Description of Mbarara Regional Referral Hospital.
Mbarara Regional Referral Hospital (MRRH) is located in Uganda in Mbarara municipality (286 km southwest of Kampala). It is a 400-bed hospital that serves a population of approximately 1.2–2.5 million people from surrounding districts in Southwestern Uganda. It also serves as a teaching hospital for the Faculty of Medicine at the Mbarara University of Science and Technology.
The hospital has a radiology department with X-ray and ultrasound facilities but no capability for computed tomography. It also has a clinical laboratory in which basic investigations, including blood and CSF cultures, can be performed. The laboratory is enrolled in the International Organisation for Standardisation and maintained satisfactory performance throughout the study period.
Patient recruitment.
From May of 2010 to August of 2010, patients were enrolled if they were ≥ 18 years of age, admitted to the medical ward, and clinically diagnosed with meningitis by the admitting medical team. Patients were excluded if they required triage to a surgical or obstetrics and gynecology ward or could not undergo lumbar puncture.
Data collection and definitions.
At the time of enrollment, consent was obtained from the patient or a surrogate (an accompanying family member or friend) if the patient could not provide consent. The study team observed patients until discharge or death, but the admitting medical team was responsible for clinical management. We retrieved the following data for each patient: demographics; examination findings, including admission vital signs; human immunodeficiency virus (HIV) serology status; and in-hospital and 30-day mortality.
Blood cultures were obtained through aseptic inoculation of 10 mL blood into two aerobic blood culture bottles before antibiotic administration. Subculturing and species identification were performed using standard methods. Bacteremia was defined as bacterial isolation from one or more blood culture bottles. Cultures growing coagulase-negative staphylococci were considered contaminated and not included in the final analyses.
CSF was evaluated through lumbar puncture for the presence of white blood cells (WBCs), glucose, protein, and POCL. Additional CSF tests included gram stain, culture, cryptococcal antigen, India ink stain, and Ziehl–Neelsen stain. An aliquot of CSF was frozen at –70°C and subsequently tested for Mycobacterium tuberculosis (MTb) by polymerase chain reaction (PCR) at Makerere University in Kampala.8 All clinical and laboratory data were provided to the attending medical team as soon as they were available.
A diagnosis of bacterial meningitis required organisms identified on CSF gram stain or culture or at least 100 WBC/μL CSF, of which ≥ 50% were polymorphonuclear leukocytes (PMNs).9 The finding of Cryptococcus by gram stain, CSF cryptococcal antigen (CRAG), or India ink stain or MTb identified by PCR excluded the diagnosis of bacterial meningitis. Proven bacterial meningitis was defined by the identification of organisms by CSF gram stain or culture or organisms compatible with bacterial meningitis cultured from blood (e.g., Streptococcus pneumoniae). Patients not diagnosed with bacterial, cryptococcal (CM) or tuberculous meningitis (TBM) were diagnosed with aseptic meningitis if ≥ 5 WBC/μL CSF were noted.
Statistics.
Consistency was statistically evaluated by calculating an R2 and Student's paired t test for POCL results. A Bland–Altman analysis was also applied. Receiver operating characteristic (ROC) curves provided predictive use of CSF POCL for meningitis diagnosis. SPSS version 16 was used for all statistical analyses. Significance level was set to P < 0.05.
Ethics statement.
The University of Virginia and Mbarara University of Science and Technology Institutional Review Boards approved the study before initiation.
Results
Validation.
There was no significant difference between POCL test 1 concentration (mean = 2.37, SD ± 1.93, range = 0.69–12.2 mmol/L) and POCL test 2 concentration (mean = 2.35, SD ± 1.93, range = 0.69–11.7 mmol/L, mean difference = 0.01 mmol/L, limits of agreement = 2 SD ± 0.04 mmol/L; P = 0.375). A Bland–Altman analysis showed no relationship between test differences and averaged test result, suggesting that small test differences remained consistent across the spectrum of lactate concentrations (R2 = 0.00; P = 0.99).
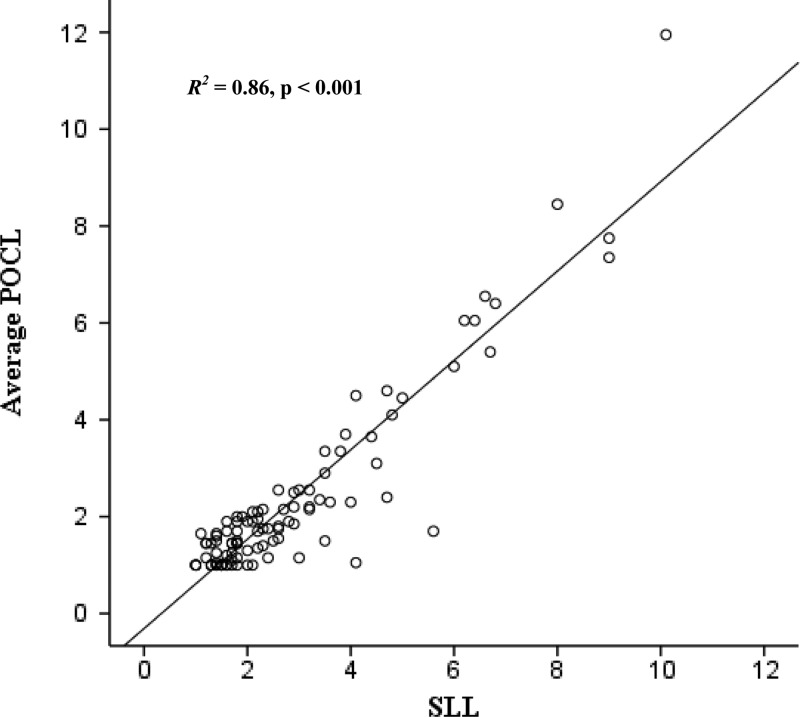
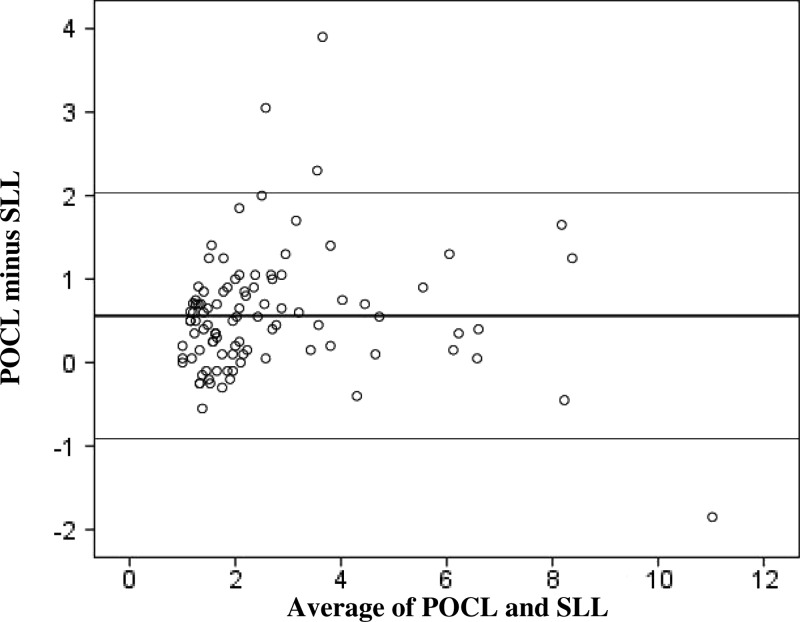
There was a strong linear correspondence between POCL and SLL test results (R2 = 0.86; P < 0.001) (Figure 1). Student's paired t test indicated significantly slightly higher SLL (mean = 2.89, SD ± 1.91, range = 1.00–10.10 mmol/L) compared with average POCL concentrations (mean = 2.33, SD ± 1.92, range = 0.69–11.95 mmol/L, mean difference = 0.56 mmol/L, limits of agreement = 2 SD ± 1.47 mmol/L; P < 0.001). There was no relationship between test differences and average lactate concentration, suggesting that test differences remained consistent across the spectrum of lactate concentrations (R2 = 0.00; P = 0.77) (Figure 2).
Figure 1.
Scatter plot with regression line of average POCL and SLL results (in millimoles per liter) for CSF samples submitted to the clinical laboratory at the University of Virginia.
Figure 2.
Bland and Altman plot of POCL and SLL results (in millimoles per liter) for CSF samples submitted to the clinical laboratory at the University of Virginia.
Population characteristics of patients evaluated in Mbarara, Uganda.
An equal proportion of men (72 of 145, 50%) and women (73 of 145, 50%) were studied. The mean (± SD) age was 37 ± 13 years (range = 18–80). The majority (116 of 143, 81%) were HIV-infected, and their median CD4+ T-cell concentration was 78/μL (interquartile range [IQR] = 21–177). Of the 116 HIV-infected patients, 40 (35%) patients were receiving antiretroviral therapy, and 67 (58%) patients were receiving Pneumocystis jiroveci prophylaxis (Table 1).
Table 1.
Demographics and clinical characteristics of patients enrolled with clinical meningitis
| Combined (N = 145)* | Bacterial meningitis (N = 17) | CM (N = 35) | TBM (N = 32) | Aseptic meningitis (N = 63) | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years), mean (SD) | 37 (13) | 38 (12) | 35 (11) | 40 (16) | 36 (12) |
| Female, n (%) | 73 (50) | 12 (71) | 16 (46) | 14 (44) | 32 (51) |
| HIV infected, n (%) | 116 (81) | 11 (65) | 34 (100) | 25 (81) | 48 (76) |
| Exam findings | |||||
| Temperature (°C), mean (SD) | 37.7 (1.2) | 38.1 (1.4) | 37.4 (1) | 37.8 (1.1) | 37.8 (1.2) |
| Abnormal temperature, n (%) | 64 (44) | 9 (47) | 10 (29) | 15 (47) | 31 (49) |
| Heart rate (beats/minute), mean (SD) | 98 (21) | 106 (22) | 90 (18) | 97 (21) | 100 (22) |
| Abnormal heart rate, n (%) | 78 (55) | 12 (71) | 15 (43) | 14 (47) | 37 (60) |
| Respiratory rate (breaths/minute), mean (SD) | 23 (8) | 30 (13) | 19 (3) | 22 (6) | 24 (8) |
| Abnormal respiratory rate, n (%) | 57 (41) | 12 (71) | 5 (15) | 10 (33) | 30 (50) |
| SBP (mmHg), mean (SD) | 109 (23) | 109 (20) | 113 (16) | 101 (23) | 110 (25) |
| DBP (mmHg), mean (SD) | 68 (19) | 72 (16) | 74 (13) | 63 (16) | 67 (23) |
| GCS, mean (SD) | 12 (3) | 9 (4) | 13 (3) | 13 (2) | 12 (3) |
| Meningismus, n (%) | 64 (44) | 13 (77) | 18 (51) | 10 (31) | 25 (40) |
| Kernigs sign, n (%) | 24 (18) | 6 (43) | 8 (23) | 4 (13) | 8 (14) |
| Laboratory findings | |||||
| WBC/μL, median (IQR) | 5,000 (3,275–7,600) | 6,800 (4,700–9,250) | 3,550 (2,800–4,400) | 4,200 (2,800–8,200) | 5,400 (3,300–8,900) |
| Abnormal WBC, n (%) | 42 (47) | 2 (15) | 13 (72) | 8 (53) | 20 (44) |
| HB, mean (SD) | 10 (4) | 11 (3) | 10 (3) | 7.7 (3.6) | 10 (5) |
| Glucose (mg/dL), mean (SD) | 119 (78) | 154 (68) | 109 (61) | 122 (107) | 114 (72) |
| Whole-blood lactate (mmol/L), mean (SD) | 3.9 (2.1) | 4.2 (1.9) | 3.8 (2.0) | 3.6 (1.3) | 3.9 (2.5) |
| Malaria, n (%) | 10 (7) | 0 (0) | 2 (6) | 2 (6) | 6 (10) |
| CSF findings | |||||
| Opening pressure (mmHg) | 22 (12) | 23 (11) | 30 (12) | 14 (7.6) | 21 (12) |
| CSF WBC/μL, median (IQR) | 10 (5–55) | 500 (150–2,240) | 15 (10–40) | 10 (5–30) | 10 (5–40) |
| CSF glucose (mg/dL), mean ± SD | 46 (35) | 31 (37) | 34 (24) | 59 (46) | 50 (31) |
| CSF protein (mg/dL), mean ± SD | 44 (36) | 88 (26) | 40 (33) | 31 (27) | 40 (35) |
| CSF lactate (mmol/L), mean ± SD | 5.5 (4.5) | 14.2 (6.2) | 4.5 (1.8) | 3.7 (2.2) | 4.7 (2.9) |
DBP = diastolic blood pressure; GCS = Glasgow coma score; HB = hemoglobin; SBP = systolic blood pressure.
Two patients had TBM and CM.
Meningitis diagnoses and associated mortality.
Of 145 patients tested, 63 (43%) patients were diagnosed with aseptic meningitis. CM and TBM were diagnosed in 35 (24%) and 32 (22%) patients, respectively. There were two (1%) patients diagnosed with both CM and TBM. None of the patients ultimately diagnosed with TBM by PCR had a positive CSF Ziehl–Neelsen stain. Of 17 (12%) patients diagnosed with bacterial meningitis, a microbiological diagnosis was available in 8 (47%) patients. In patients with bloodstream infections (N = 23, 16%), Staphylococcus aureus was the most frequent isolate (N = 17, 74%) followed by S. pneumoniae (N = 4, 17%) and Salmonella species (N = 2, 9%). Cryptococcus species was most frequently isolated from CSF cultures (N = 12, 67%) followed by S. pneumoniae (N = 3, 17%), gram negatives not further speciated (N = 2, 11%), and Neisseria meningitidis (N = 1, 6%). Not all patients with bacteremia had a diagnosis of bacterial meningitis.
The in-hospital mortality for patients with and without bacterial meningitis was 58.8% (10 of 17) and 28.1% (36 of 128), respectively. The average time to death for patients with bacterial meningitis was 3.0 ± 1.8 days (range = 1–6) compared with 7.3 ± 6.6 days (range = 1–27) for non-bacterial meningitis cases. Within the non-bacterial meningitis group, the in-hospital mortality was 25.7% (9 of 35) for CM and 28.1% (9 of 32) for TBM; 30-day mortality data were available for 87.6% (127 of 145) of patients. Of these patients, patients with bacterial meningitis had a 30-day mortality of 73.3% (11 of 15) compared with 48.2% (54 of 112) for those patients without bacterial meningitis. The 30-day mortality for CM and TBM was 51.9% (14 of 27) and 46.7% (14 of 30), respectively.
Predictive value of CSF POCL for bacterial meningitis.
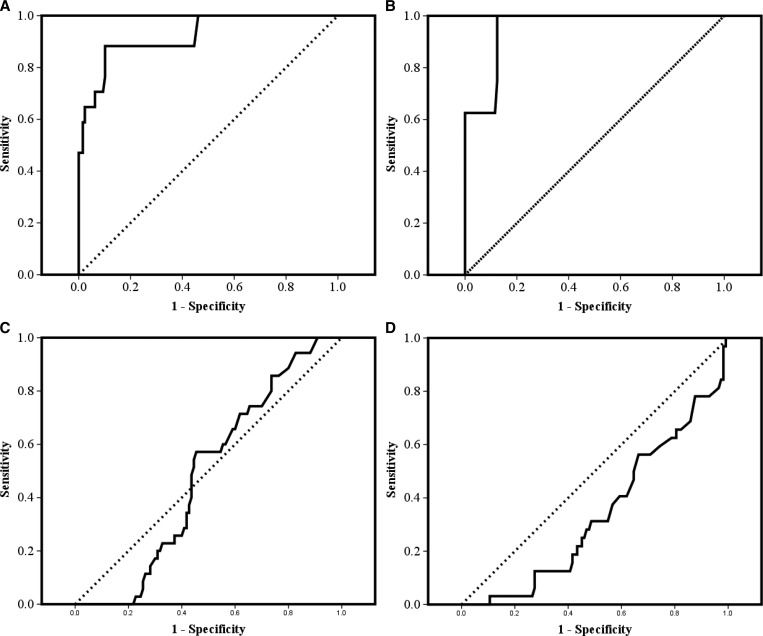
The area under the ROC curve (AUC) for the prediction of bacterial meningitis by CSF POCL was 0.92 (95% confidence interval [CI] = 0.85–0.99, P < 0.001) (Figure 3A). A CSF POCL concentration of 7.7 mmol/L provided 88% sensitivity and 90% specificity for the diagnosis of bacterial meningitis. No cases of bacterial meningitis occurred with a CSF POCL < 3.75 mmol/L (100% sensitivity). Every patient with a CSF POCL > 13.5 mmol/L had bacterial meningitis (100% specificity).
Figure 3.
ROC curves, with sensitivity on the y axis and 1-specificity on the x axis, for prediction of (A) bacterial meningitis, (B) proven bacterial meningitis, (C) CM, and (D) TBM on the basis of different CSF POCL concentrations.
CSF POCL was also an excellent predictor of proven bacterial meningitis (AUC = 0.95, 95% CI = 0.9–1.0, P < 0.001) (Figure 3B). A CSF POCL concentration of 7.7 mmol/L provided 100% sensitivity and 85% specificity for the diagnosis of culture-positive bacterial meningitis. No cases of proven bacterial meningitis occurred with a CSF LA < 7.7 mmol/L (100% sensitivity). Every patient with a CSF LA > 17 mmol/L had culture-positive bacterial meningitis (100% specificity). CSF lactate was not informative regarding the diagnosis of CM (AUC = 0.48, 95% CI = 0.39–0.58, P = 0.73) (Figure 3C). However, there was a statistically significant inverse relationship between CSF POCL and TBM (AUC = 0.34, 95% CI = 0.24–0.44, P = 0.006) (Figure 3D).
Discussion
For the first time, we have shown that a handheld POCL monitor can accurately measure CSF lactate and predict bacterial meningitis. Our data suggest that bacterial meningitis is a severe disease in our setting, with approximately 73% of patients succumbing to the infection. These deaths occurred precipitously, with an average time to death of only 3 days. The ability to rapidly diagnose bacterial meningitis potentially means faster time to antibiotic administration and better survival. Additionally, excluding bacterial meningitis may prevent unnecessary exposure to antibiotics and their inherent costs and toxicities. In this light, the ability of POCL to predict bacterial meningitis is an important and clinically useful finding.
We found that a CSF POCL value of 3.75 mmol/L provided the most sensitive (100%) marker for bacterial meningitis, because no patient with a CSF POCL < 3.75 mmol/L had bacterial meningitis. However, the low specificity of this value (53.5%) suggests that it is not the most useful clinical indicator to diagnose bacterial meningitis. Instead, a CSF POCL of 7.7 mmol/L provides a more clinically useful cutoff, because this value was the most sensitive (88%) and specific (90%) for the diagnosis of bacterial meningitis. Other studies have found values ranging from 3.5 to 4.2 mmol/L as the best sensitivity and specificity for meningitis.6,10–12 There was a slight discrepancy between POCL and SLL values in our study; this result is not likely to be clinically meaningful, because the mean difference was only approximately 0.5 mmol/L.
In Uganda, patients presenting with clinical meningitis are often infected with HIV, which means that, along with bacterial causes, CM and TBM are important considerations.13 The ability to identify these etiologies is important to guide specific therapy. Although POCL showed no ability to identify Cryptococcus, we found an inverse relationship between POCL concentration and a diagnosis of TBM. In the case of a protracted course of meningitis, a high POCL may make TBM less likely than CM. Conversely, a low POCL may indicate TBM when other causes, such as CM, have been ruled out. We also found that Ziehl-Neelsen staining of CSF was of no benefit in the diagnosis of TBM, because 0 of 32 patients with TBM had a positive stain.
The POCL monitor is easy to use and portable, which means it can be used in settings where laboratory infrastructure is limited and clinical personnel are scarce. For example, in much of sub-Saharan Africa, it is not possible to obtain timely and accurate CSF chemistries, and microscopic findings of gram stain and cell count may be inaccurate.3,13 Use of CSF POCL testing is particularly attractive, because the monitor and reagents are relatively cheap compared with a fully equipped laboratory; additionally, refrigeration is not required, the testing only requires one drop of CSF, the results are available in only 60 seconds, and testing can be done by anyone after a brief training session. In such settings, discrimination of bacterial versus non-bacterial meningitis could be greatly augmented by CSF POCL testing, which could result in a reduction of already limited expenditures and antibiotics.
ACKNOWLEDGMENTS
We appreciate W. Michael Scheld's review of the manuscript.
Footnotes
Financial support: A.M., R.B., and C.C.M. received fellowships from the Pfizer Initiative in International Health at the University of Virginia to support this work. The Pfizer Initiative in International Health at the University of Virginia was conceived to fund exchange programs of post-doctoral fellows and students between the University of Virginia and several international partners to conduct research on global health issues. The major purpose of this program is to foster and enhance bidirectional research training for treating infectious diseases like acquired immunodeficiency syndrome, tuberculosis, and malaria. Pfizer provided funds to promote the Initiative but has no role in the planning or execution of research protocols, including the study described in our manuscript. An independent board at the University of Virginia determines the research proposals that are funded.
Authors' addresses: Albert Majwala, Conrad Muzoora, and L. Anthony Wilson, Mbarara Regional Referral Hospital, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda, E-mails: majalb2k@yahoo.com, conradmuzoora@yahoo.com, and tonywislon@gmail.com. Rebecca Burke, Department of Medicine, Duke University School of Medicine, Durham, NC, E-mail: rebeccaburke00@gmail.com. William Patterson, Department of Laboratory Medicine, University of Virginia School of Medicine, Charlottesville, VA, E-mail: wmp4c@virginia.edu. Relana Pinkerton and Christopher C. Moore, Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia, Charlottesville, VA, E-mails: rcp3w@virginia.edu and ccm5u@virginia.edu.
References
- 1.Ziai WC, Lewin JJ., III Advances in the management of central nervous system infections in the ICU. Crit Care Clin. 2006;22:661–694. doi: 10.1016/j.ccc.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 2.van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849–1859. doi: 10.1056/NEJMoa040845. [DOI] [PubMed] [Google Scholar]
- 3.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 4.Ross D, Rosegay H, Pons V. Differentiation of aseptic and bacterial meningitis in postoperative neurosurgical patients. J Neurosurg. 1988;69:669–674. doi: 10.3171/jns.1988.69.5.0669. [DOI] [PubMed] [Google Scholar]
- 5.Guerra-Romero L, Tauber MG, Fournier MA, Tureen JH. Lactate and glucose concentrations in brain interstitial fluid, cerebrospinal fluid, and serum during experimental pneumococcal meningitis. J Infect Dis. 1992;166:546–550. doi: 10.1093/infdis/166.3.546. [DOI] [PubMed] [Google Scholar]
- 6.Leib SL, Boscacci R, Gratzl O, Zimmerli W. Predictive value of cerebrospinal fluid (CSF) lactate level versus CSF/blood glucose ratio for the diagnosis of bacterial meningitis following neurosurgery. Clin Infect Dis. 1999;29:69–74. doi: 10.1086/520184. [DOI] [PubMed] [Google Scholar]
- 7.Huy NT, Thao NT, Diep DT, Kikuchi M, Zamora J, Hirayama K. Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: a systemic review and meta-analysis. Crit Care. 2010;14:R240. doi: 10.1186/cc9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muhumuza J, Asiimwe BB, Kayes S, Mugyenyi R, Whalen C, Mugerwa RD, Boom H, Eisenach KD, Joloba ML. Introduction of an in-house PCR for routine identification of M. tuberculosis in a low-income country. Int J Tuberc Lung Dis. 2006;10:1262–1267. [PubMed] [Google Scholar]
- 9.Scarborough M, Thwaites GE. The diagnosis and management of acute bacterial meningitis in resource-poor settings. Lancet Neurol. 2008;7:637–648. doi: 10.1016/S1474-4422(08)70139-X. [DOI] [PubMed] [Google Scholar]
- 10.Bailey EM, Domenico P, Cunha BA. Bacterial or viral meningitis? Measuring lactate in CSF can help you know quickly. Postgrad Med. 1990;88:217–219. 223. doi: 10.1080/00325481.1990.11716403. [DOI] [PubMed] [Google Scholar]
- 11.Cameron PD, Boyce JM, Ansari BM. Cerebrospinal fluid lactate in meningitis and meningococcaemia. J Infect. 1993;26:245–252. doi: 10.1016/0163-4453(93)95253-f. [DOI] [PubMed] [Google Scholar]
- 12.Genton B, Berger JP. Cerebrospinal fluid lactate in 78 cases of adult meningitis. Intensive Care Med. 1990;16:196–200. doi: 10.1007/BF01724802. [DOI] [PubMed] [Google Scholar]
- 13.Trachtenberg JD, Kambugu AD, McKellar M, Semitala F, Mayanja-Kizza H, Samore MH, Ronald A, Sande MA. The medical management of central nervous system infections in Uganda and the potential impact of an algorithm-based approach to improve outcomes. Int J Infect Dis. 2007;11:524–530. doi: 10.1016/j.ijid.2007.01.014. [DOI] [PubMed] [Google Scholar]