Abstract
Autochthonous human cases of leishmaniasis in the United States are uncommon. We report three new cases of cutaneous leishmaniasis and details of a previously reported case, all outside the known endemic range in Texas. Surveys for enzootic rodent reservoirs and sand fly vectors were conducted around the residences of three of the case-patients during the summer of 2006; female Lutzomyia anthophora sand flies were collected at a north Texas and southeast Oklahoma residence of a case-patient, indicating proximity of a suitable vector. Urban sprawl, climatologic variability, or natural expansion of Leishmania mexicana are possible explanations for the apparent spread to the north and east. Enhanced awareness among healthcare providers in the south central region of the United States is important to ensure clinical suspicion of leishmaniasis, diagnosis, and appropriate patient management.
Introduction
The leishmaniases are a complex of parasitic diseases that cause significant health problems on almost all continents. Clinically, there are three major syndromes, cutaneous, mucocutaneous, and visceral, depending largely on the species of Leishmania involved and immune response of the human host. With the apparent exception of the Indian subcontinent, most transmission cycles are zoonotic, with many species of rodents, wild and domestic canids, and a variety of other mammals serving as reservoir hosts and phlebotomine sand flies acting as vectors.1
Autochthonous human cases of leishmaniasis in the United States are uncommon, with only 29 cases reported from approximately 1903 through 1996.2 All were in Texas, primarily in the southern and central areas of the state. In 2000, Maloney and others diagnosed a case acquired in Brenham, Texas, which is well east of previous cases.3 More recently, Wright and others summarized a cluster of nine cases in the Dallas-Fort Worth metroplex and surrounding counties, well to the east-northeast of the northernmost case previously reported in Albany, Texas.4 Clinically, most cases had 1–5 localized cutaneous lesions of a few months duration. Specific identification using isoenzyme analysis and/or polymerase chain reaction (PCR) was performed on 10 of these 39 cases; all were determined to be Leishmania mexicana.
In the United States, L. mexicana exists as a zoonosis, with three species of woodrats, Neotoma micropus, Neotoma albigula, and Neotoma floridana, serving as reservoir hosts.5–9 Based on laboratory transmission studies and the isolation of L. mexicana from field-collected specimens, Lutzomyia anthophora sand flies, a nest associate of woodrats, is the only known enzootic vector of L. mexicana in the United States.10–12 Both of these reservoir and vector species are nocturnal.
We report three additional cases of cutaneous leishmaniasis in humans who had no foreign travel history. We also present details of a previously reported case that extend the known range of human disease in the United States and the results of field surveys to document the enzootic cycle, and suggest explanations for the apparent expansion of the geographic range of human disease.
Case Report
Case 1.
A 26 year-old man from a small community in McCurtain County in southeastern Oklahoma first noted a lesion on the periorbital area of his right cheek in December 2003. In January 2004, he sought medical care from a local physician, who excised the entire lesion measuring 1.0 × 0.7 × 0.4 cm and submitted the tissue to a pathologist in Oklahoma City. The pathologist noted that an infectious process was likely, but initially ruled out Leishmania, acid-fast, and fungal organisms. Histopathologic findings were revisited by the pathologist when a similar case (case 3) was evaluated from the same community. A secondary review at the Centers for Disease Control and Prevention (CDC) (Atlanta, GA) confirmed a diagnosis of leishmaniasis, the first known from the state. The case-patient received no anti-parasitic treatment and there has been no recurrence. Case-patient 1 participated in an initial interview but did not allow access to his property for an ecologic survey.
Case 2.
In June 2005, a 74 year-old woman from Lamar County, Texas, noted an inflammatory nodule on her left eyelid, for which she sought ophthalmologic care the next month. When she returned for follow-up 10 days later, the lesion had crusted with a central scab and measured 1.0 × 1.5 cm on an excisional biopsy specimen. The biopsy tissue was sent to the Texas Department of State Health Services Laboratory where leishmaniasis was diagnosed by histopathologic examination. Treatment consisted of heat packs applied to the affected area.
The residence of case-patient 2 was in a rural area a few miles south of Paris, Texas. Vegetation in the area consisted of mixed hardwoods and pasture. Piles of debris and many abandoned vehicles were observed near the house, and there were several unused chicken coops containing loose feed and two enclosures with chickens near the rear of the house.
Case 3.
In December 2005, a 73 year-old man from McCurtain County, Oklahoma, noted two eruptive skin lesions on his right forearm. When the lesions failed to heal, he sought care in February 2006, from the local physician, who had treated case-patient 1. The cutaneous lesions measured 1.8 × 1.0 × 0.6 cm and 2.5 × 1.5 × 0.9 cm and were excised and submitted to the same pathologist who performed the histopathologic evaluation for case-patient 1. The pathologist noted the presence of intracytoplasmic organisms suggestive of Leishmania. Recalling that case-patient 3 lived in the same small community as case-patient 1, he retrieved the slides for case-patient 1 for another review and reconsidered cutaneous leishmaniasis. He then contacted the Oklahoma State Department of Health (OSDH) to report two suspected cases of cutaneous leishmaniasis. Slides from the Oklahoma cases were submitted to the CDC for diagnostic review; the presence of Leishmania organisms was confirmed in tissue biopsy specimens from each case-patient.
Case-patient 3 resided on a large lot that housed several horses, and included a corral, barns, sheds, and outbuildings. An ornamental pond was located near the home. A woodlot of hardwoods adjoined the property. His extended family lived on a nearby lot where his granddaughter raised rabbits. Case-patient 3 was retired, but remained actively involved in rearing cattle on three parcels of land located a few miles from his residence.
Case 4.
This case was patient 9 in the report of Wright and others,4 which they reported from Tarrant County, Texas. In December 2005, an elementary school-age girl from Collin County, Texas, showed development of three skin lesions, two facial and one upper extremity. Medical care began in January 2006, and included topical and oral antibiotics, topical antifungal drugs, and topical corticosteroids, but no clinical improvement was noted. A biopsy in April 2006, yielded a histopathologic diagnosis of leishmaniasis and a specific determination of L. mexicana was made by culture at the CDC. Screening by PCR using forward primer 13A and reverse primer M1.19 confirmed that the parasites belonged to the L. mexicana complex.
The residence of case-patient 4 was built in 2001 and was located in an urban-rural interface in one of the fastest growing counties in Texas approximately 38 miles north of Dallas. It is on a lot measuring approximately 5 acres with a dry creek bed bordered by mixed hardwoods and surrounded by fields planted to cotton. Domestic animals and livestock on the property included a small dog, two cats, and longhorn cattle that graze in the creek bed. Large rodent burrows were observed along the creek bed. An animal enclosure containing rabbits, sheep, and goats was also located near the residence.
Surveillance for additional human cases.
In April 2006, an electronic advisory was issued through the OSDH Health Alert Network to physicians in family practice, internal medicine, and pediatric medicine located in McCurtain, Pushmataha, and Choctaw Counties informing them of the recent recognition of endemic leishmaniasis in southeast Oklahoma and requesting reporting of suspect cases to the OSDH. In June 2006, a letter describing the clinical aspects of cutaneous leishmaniasis, including a website link showing a typical lesion, was sent to pathologists and dermatologists over a larger region of Oklahoma. The health departments of the neighboring states of Arkansas and Louisiana were contacted by the Oklahoma State Epidemiologist and queried regarding any known case reports of leishmaniasis. No additional cases were identified in southeastern Oklahoma, or the adjacent states of Arkansas or Louisiana.
Rodent collections.
A total of 303 Sherman live traps (H.B. Sherman Traps, Inc., Tallahassee, FL) baited with rolled oats were set near burrows or other likely rodent habitat at four locations: the residence and adjacent pastures of case-patient 3, the residence of case-patient 2, two pastures belonging to case-patient 2, and a barn and adjacent riparian bottomland along the Red River during June 12–16, 2006. The primary species of interest were the eastern woodrat, N. floridana, and the hispid cotton rat, Sigmodon hispidus, an incidental host of L. mexicana in the Yucatan Peninsula and a species commonly found in pastureland. A total of only 13 rodents were captured. A single N. floridana was collected in the barn near the Red River in Lamar County. The remaining rodents were house mice, Mus musculus and mice in the genus Peromyscus. No S. hispidus were collected. The woodrat was negative by PCR for Leishmania using the genus-specific primers 13A and 13B.13
Sand fly collections.
On the same dates in mid-June, 31 incandescent light traps (Model 1012; John W. Hock Co., Gainesville, FL) and blacklight traps (Model 1212; John W. Hock Co.) were set on the residential property of the four case-patients to survey for sand flies. Light traps were placed near residences, either near favorable rodent habitat or near domestic animal locations (rabbits, chickens, horses), to collect sand flies. No traps were placed inside homes. The vector traps were operational throughout the night with insect collections retrieved the next morning. Sand flies were identified by using the key of Young and Perkins.14 A single female Lu. anthophora was collected at a rabbit hutch near the residence of case-patient 3.
A total of 12 ABC light traps (Clarke Mosquito Control, Roselle, IL) were placed at the residence of case-patient 4 in Collin County on three separate nights (June 28, July 12, and September 19, 2006). A total of 23 sand flies were collected during the three nights: 3 female Lu. anthophora, 12 female and 1 male Lutzomyia vexator, and 7 female Lutzomyia spp., which were probably Lu. vexator.
Discussion
In the United States, there appears to be a trend of diagnosing human leishmaniasis with increasing frequency. From 1903 through 1996, only 29 cases were reported. For the eight-year period from 2000 through 2007, an additional 13 cases have now been diagnosed.
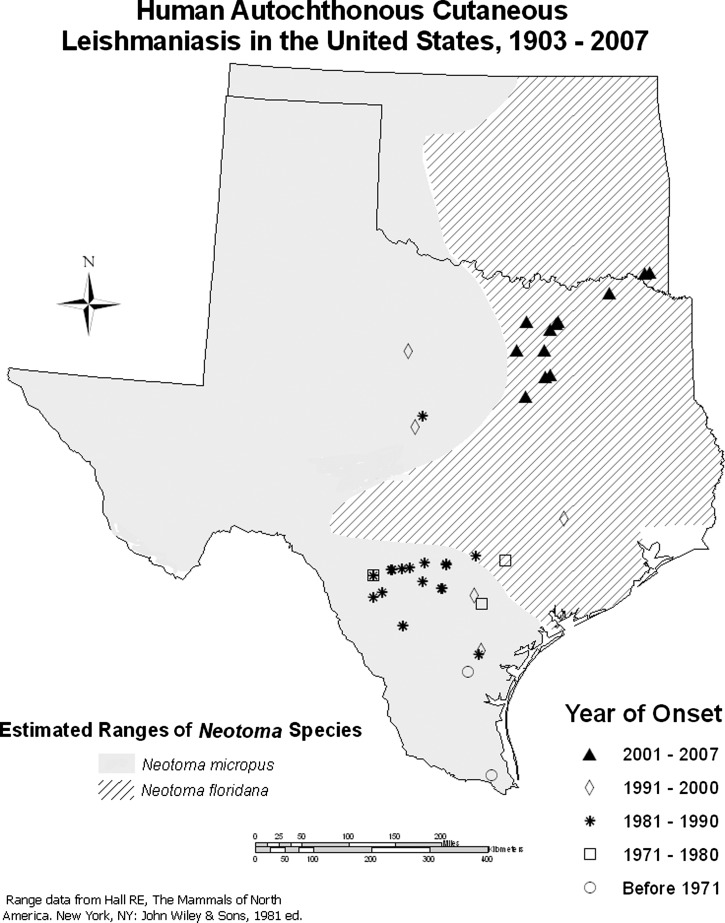
There also appears to be a northeasterly geographic trend in the occurrence of human cases (Figure 1). The first recognized case in the United States was in a 64 year-old woman given a diagnosis of leishmaniasis in the late 1960s, but who probably acquired the infection in approximately 1903 at San Benito in Cameron County, the southernmost county in Texas.15 A second case in Alice, Texas, approximately 130 miles further north, was acquired in early 1942.16 During the 1970s and 1980s, cases were diagnosed around San Antonio, west to Uvalde, Texas, and south to northern Mexico.17–23 In 1988, a case was reported in the central Texas city of Brownwood and in 1992 in nearby Brookesmith. In November 1994, the northernmost case to that date was recognized in Albany, Texas. Until that time, all reported cases were within the range of the southern plains woodrat N. micropus. The four cases in northeastern Texas and southeastern Oklahoma represent a further expansion of autochthonous human leishmaniasis to the north and east. From the nearest case in Collin County, Texas, the case in Lamar County was approximately 75 miles away. The two cases in southeastern Oklahoma were approximately 58 miles from the case in Lamar County, Texas. All of these newly reported cases are in the range of N. floridana.
Figure 1.
Historic occurrence of autochthonous human cases of leishmaniasis in Texas and Oklahoma.
Our findings in this report were limited by the fact that diagnostic testing for case-patients 1–3 was based on histopathologic analysis alone. Although culture or PCR testing to identify Leishmania species were not obtained, the clinical presentation, epidemiology, and outcome of these cases is consistent with L. mexicana infection. Another limitation was the inability to document L. mexicana in a rodent or sand fly specimen collected in the relative vicinity of the likely exposure of the case-patients. The geographic distribution of Leishmania is known to be highly focal, both spatially and temporally, and the timing of our epidemiologic investigation was delayed by several months to a few years from each case's exposure period, and mismatched to the seasonality of L. mexicana transmission.
Pavlovsky discussed factors that determine the distribution and spread of zoonotic diseases, including a number that are rodent-borne, in the former Soviet republics.24 More recently, Shaw reviewed reasons for the increase in human cases of leishmaniasis worldwide.25 Of the factors these authors cite, the increasing frequency of diagnoses and expansion in geographic range in the southern United States may have at least three explanations: increased clinical suspicion; human intrusion into established foci; or introduction of one or more missing components, reservoir, vector, or pathogen, of the enzootic cycle.
It is unlikely that increased clinical suspicion contributed to the recognition of the reported cases, but it cannot be ruled out. A diagnosis of leishmaniasis for case-patient 1 was delayed for more than one year and would have been dismissed entirely if not for the serendipitous occurrence of case-patient 3 in the same Oklahoma community and biopsy evaluation by the same pathologist. Although heightened clinical awareness may be anticipated among physicians in Texas, disease occurrence is rare. Case-patient 4 received multiple treatment modalities before a diagnosis of cutaneous leishmaniasis was made.
Increased exposure to the parasite could result from human intrusion into environments in which it exists. This factor may account for case-patient 4 in our series. The residence of this case-patient was built in 2001 on what formerly was farmland with a limited area of riparian forest. This region is an area of rapid urban development, but with a trend to preserve the natural surroundings and eco-systems around the homes. The long-standing presence of riparian vegetation, suitable habitat for N. floridana, and the collection of Lu. anthophora and Lu. vexator in the immediate vicinity suggests that this was an established enzootic focus of L. mexicana transmission.
In the absence of urbanization, increased contact could occur by creating environmental conditions that draw the enzootic cycle into peridomestic surroundings. Case-patients 2 and 3 may be representative. Both of the residences of these case-patients were in areas experiencing little or no growth and with stable habitat. Case-patient 2 had extensive debris piles and discarded vehicles that are attractive harborage for woodrats around the residence. In addition, there were suitable blood meal hosts for Lu. anthophora near both case-patient residences. At the home of case-patient 2, there were chickens near the house. McHugh and others reported the presence of domestic fowl at other case residences in southern Texas.2 Case-patient 3 had a rabbit in a hutch near the residence and the only female Lu. anthophora collected during the mid-June surveys was trapped at the hutch. The presence of rabbits at residences of case-patients has been noted.2
Increased contact could also occur if resident populations of hosts and/or vectors were drawn to peridomestic habitats during periods of environmental stress, such as drought conditions. In Colombia and Brazil, drought has been found to accompany increased numbers of human leishmaniasis cases.26–28 In the six months preceding the onset of our four cases, average monthly rainfall was decreased compared with the 30-year historic averages. Six-month precipitation departures ranged from 5.80 inches (case-patient 2) to 13.86 inches (case-patient 4) below the expected rainfall for the respective location. Three of the four case-patients had symptom onsets during June–December 2005. In a 2008 report, the National Weather Service described 2005 as the second driest calendar year on record for southeastern Oklahoma. The effects of climatic conditions on Lu. anthophora are unknown. However, Bradley and others documented a decrease in populations of N. micropus in response to drought and a rapid recovery to pre-drought levels once rainfall returned to normal levels.29 A similar decrease in populations of N. floridana may also result from drought conditions. As rodent hosts become less available, sand flies may potentially seek blood meals from novel or nonpreferred sources, such as humans or peridomestic animals.
Climatic factors may also lead to shifts in the range of vector and reservoir species. Analyses by Gonzalez and others30 using ecological niche models of sand fly vectors and Neotomae rodent reservoirs predict that the range of all confirmed vector and reservoir species of L. mexicana will continue a northward expansion outside of its present range spurred by climate change. As a result of this expansion, the numbers of persons exposed to L. mexicana will increase.30
The introduction and spread of mosquito vectors such as Aedes albopictus in North America are well documented.31 Unfortunately, the historic and present distribution of sand flies is imperfectly known and probably reflects more the distribution of collectors than of the flies. Until 1995, Lu. anthophora was believed to have a distribution limited to southern and western Texas.14,32 The collection of Lu. anthophora near Tucson was an extension of its range of over 528 miles to the west-northwest.33 The Lu. anthophora collected near the residence of case-patient 4 in Collin County are approximately 290 miles from the nearest previously known collections near San Antonio, Texas. They represent a new county record, but because this was the first attempt at collection in the county, it does not necessarily mean they are new to the area. Likewise, the collection of Lu. vexator is a new county record, but this species was previously collected in central Oklahoma. The collection of the single Lu. anthophora female in McCurtain County, Oklahoma, represents a new county and state record. The findings of our vector surveys serve to establish the presence of a suitable vector for leishmaniasis in the vicinity of the cases; however, the trapping yield was low. Another limitation was the length of time that elapsed between the likely period of exposure of case-patients and timing of the insect trapping.
Although the contributing ecologic factors for emergence of L. mexicana into a new geographic focus cannot be elucidated, it is clear that awareness among the medical and public health sectors in the south central region of the United States needs to be enhanced. The location of the cases in southeastern Oklahoma is only a short distance from Arkansas and Louisiana, and the potential for expansion of L. mexicana in northerly and easterly directions exists. Increased public health surveillance is desirable to further monitor the emergence of autochthonous leishmaniasis. Leishmaniasis is not on the list of nationally reportable conditions. Diagnosis of leishmaniasis is reportable to public health authorities in Texas, but not in Oklahoma.
Physicians need to be aware of the possibility of cutaneous leishmaniasis in soldiers and travelers returning from disease-endemic countries, as well as residents of Texas, Oklahoma, and surrounding areas. The CDC offers diagnostic laboratory support and a Practical Guide for Laboratory Diagnosis of Leishmaniasis is accessible on the CDC web site.34 The choice of treatment depends on the Leishmania species, number, size and location of cutaneous lesions, and the presence of any underlying immunosuppressive conditions. Localized cutaneous leishmaniasis is not a life-threatening condition. Therefore, therapy selection needs to balance the degree of morbidity against the potential side effects of treatment options. Treatment regimens recommended by the CDC and the World Health Organization for L. mexicana include local therapy options of topical paramomycin, thermotherapy or intralesional administration of pentavalent antimonial drugs, or oral treatment with ketoconazole or miltefosine.35,36 Whenever leishmaniasis is suspected or diagnosed in a resident of the United States who does not have foreign travel as a risk factor, healthcare providers are encouraged to contact their local or state health department for epidemiologic investigation.
ACKNOWLEDGMENTS
We thank the staff of the United States Air Force School of Aerospace Medicine for their technical assistance in vector collection and identification; Dr. William Caire (University of Central Oklahoma, Edmond, OK) for professional expertise and kind assistance with rodent surveys and identification; Dr. Sara Kerr (University of the Incarnate Word, San Antonio, TX) for screening rodents by PCR; Katy Rich (Oklahoma State Department of Health) for assistance with map preparation.
Footnotes
Authors' addresses: Carmen F. Clarke, Dynamis, Arlington, VA, E-mail: cclarke@dynamis.com. Kristy K. Bradley, Office of the State Epidemiologist, Oklahoma State Department of Health, Oklahoma City, OK, E-mail: kristyb@health.ok.gov. James H. Wright, Tyler, TX, E-mail: docwrightdvmtx@gmail.com. Janet Glowicz, Parkland Health and Hospital System, Dallas, TX, E-mail: janet.glowicz@phhs.org.
References
- 1.Magill AJ. Leishmania species: visceral (kala-azar), cutaneous and mucosal leishmaniasis. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Seventh edition. Philadelphia, PA: Churchill Livingstone; 2010. pp. 3463–3480. [Google Scholar]
- 2.McHugh CP, Melby PC, LaFon SG. Leishmaniasis in Texas: epidemiology and clinical aspects of human cases. Am J Trop Med Hyg. 1996;55:547–555. doi: 10.4269/ajtmh.1996.55.547. [DOI] [PubMed] [Google Scholar]
- 3.Maloney DM, Maloney JE, Dotson D, Popov VL, Sanchez RL. Cutaneous leishmaniasis: Texas case diagnosed by electron microscopy. J Am Acad Dermatol. 2002;47:614–616. doi: 10.1067/mjd.2002.124606. [DOI] [PubMed] [Google Scholar]
- 4.Wright NA, Davis LE, Aftergut KS, Parrish CA, Cockrell CJ. Cutaneous leishmaniasis in Texas: a northern spread of endemic areas. J Am Acad Dermatol. 2008;58:650–652. doi: 10.1016/j.jaad.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 5.McHugh CP, Grogl M, Kerr SF. Isolation of Leishmania mexicana from Neotoma micropus collected in Texas. J Parasitol. 1990;76:741–742. [PubMed] [Google Scholar]
- 6.Merkelz RM, Kerr SF. Demographics, den use, movements, and absence of Leishmania mexicana in southern plains woodrats (Neotoma micropus) Southwest Nat. 2002;47:70–77. [Google Scholar]
- 7.Raymond RW, McHugh CP, Witt LR, Kerr SF. Temporal and spatial distribution of Leishmania mexicana infections in a population of Neotoma micropus. Mem Inst Oswaldo Cruz. 2003;98:171–180. doi: 10.1590/s0074-02762003000200002. [DOI] [PubMed] [Google Scholar]
- 8.Kerr SF, McHugh CP, Merkelz R. Short report: a focus of Leishmania mexicana near Tucson, Arizona. Am J Trop Med Hyg. 1999;61:378–379. doi: 10.4269/ajtmh.1999.61.378. [DOI] [PubMed] [Google Scholar]
- 9.McHugh CP, Thies ML, Melby PC, Yantis LD, Jr, Raymond RW, Villegas MD, Kerr SF. Short report: a disseminated infection of Leishmania mexicana in an eastern woodrat, Neotoma floridana, collected in Texas. Am J Trop Med Hyg. 2003;69:470–472. [PubMed] [Google Scholar]
- 10.Endris RG, Young DG, Perkins PV. Experimental transmission of Leishmania mexicana by a North American sand fly, Lutzomyia anthophora (Diptera: Psychodidae) J Med Entomol. 1987;24:243–247. doi: 10.1093/jmedent/24.2.243. [DOI] [PubMed] [Google Scholar]
- 11.McHugh CP, Grogl M, Kreutzer RD. Isolation of Leishmania mexicana (Kinetoplastida: Trypanosomatidae) from Lutzomyia anthophora (Diptera: Psychodidae) collected in Texas. J Med Entomol. 1993;30:631–633. doi: 10.1093/jmedent/30.3.631. [DOI] [PubMed] [Google Scholar]
- 12.McHugh CP, Ostrander BF, Raymond RW, Kerr SF. Population dynamics of sand flies (Diptera: Psychodidae) at two foci of leishmaniasis in Texas. J Med Entomol. 2001;38:268–277. doi: 10.1603/0022-2585-38.2.268. [DOI] [PubMed] [Google Scholar]
- 13.Rodgers MR, Popper SJ, Wirth DF. Amplification of kinetoplast DNA as a tool in the detection and diagnosis of Leishmania. Exp Parasitol. 1990;71:267–275. doi: 10.1016/0014-4894(90)90031-7. [DOI] [PubMed] [Google Scholar]
- 14.Young DG, Perkins PV. Phlebotomine sand flies of North America (Diptera: Psychodidae) Mosq News. 1984;44:263–304. [Google Scholar]
- 15.Simpson MH, Mullins JF, Stone OJ. Disseminated anergic cutaneous leishmaniasis. An autochthonous case in Texas and the Mexican states of Tamaulipas and Nuevo Leon. Arch Dermatol. 1968;97:301–303. doi: 10.1001/archderm.97.3.301. [DOI] [PubMed] [Google Scholar]
- 16.Stewart CD, Pilcher JF. American leishmaniasis: report of an autochthonous case. Arch Derm Syphilol. 1945;51:124–128. [Google Scholar]
- 17.Furner EB. Cutaneous leishmaniasis in Texas: report of a case and review of the literature. J Am Acad Dermatol. 1990;23:368–371. [PubMed] [Google Scholar]
- 18.Galino A, Duncan JM, Zeluff B, De Priest J, McAllister HA, Radovancevic B, Frazier OH. Leishmaniasis in a heart transplant patient. J Heart Lung Transplant. 1992;11:820–823. [PubMed] [Google Scholar]
- 19.Gustafson TL, Reed CM, McGreevy PB, Pappas MG, Fox JC, Lawyer PG. Human cutaneous leishmaniasis acquired in Texas. Am J Trop Med Hyg. 1985;34:58–63. doi: 10.4269/ajtmh.1985.34.58. [DOI] [PubMed] [Google Scholar]
- 20.Nelson DA, Gustafson TL, Spielvogel RL. Clinical aspects of cutaneous leishmaniasis acquired in Texas. J Am Acad Dermatol. 1985;12:985–992. doi: 10.1016/s0190-9622(85)70125-9. [DOI] [PubMed] [Google Scholar]
- 21.Reed CM. Autochthonous human cutaneous leishmaniasis in Texas. Border Epidemiol Bull. 1986;13:1–5. [Google Scholar]
- 22.Shaw PK, Quigg LT, Allain DS, Juranek DD, Healy GR. Autochthonous dermal leishmaniasis in Texas. Am J Trop Med Hyg. 1976;25:788–796. doi: 10.4269/ajtmh.1976.25.788. [DOI] [PubMed] [Google Scholar]
- 23.Velasco O, Savarino SJ, Walton BC, Gam AA, Neva FA. Diffuse cutaneous leishmaniasis in Mexico. Am J Trop Med Hyg. 1989;41:280–288. [PubMed] [Google Scholar]
- 24.Pavlovsky EN. Natural Nidality of Transmissible Diseases. Urbana, IL: University of Illinois Press; 1966. [Google Scholar]
- 25.Shaw J. The leishmaniases – survival and expansion in a changing world. A mini-review. Mem Inst Oswaldo Cruz. 2007;102:541–547. doi: 10.1590/s0074-02762007000500001. [DOI] [PubMed] [Google Scholar]
- 26.Cardenas R, Sandoval CM, Rodriguez-Morales AJ, Franco-Paredes C. Impact of climate variability in the occurrence of leishmaniasis in northeastern Colombia. Am J Trop Med Hyg. 2006;75:273–277. [PubMed] [Google Scholar]
- 27.Costa CH, Pereira HF, Araujo MV. Visceral leishmaniasis epidemic in the State of Piaui, Brazil, 1980–1986 [in Spanish] Rev Saude Publica. 1990;24:361–372. doi: 10.1590/s0034-89101990000500003. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RA, Wellington de Oliveira LJ, Maguire JH, Braud DH, Scholl DT. Climatic and demographic determinants of American visceral leishmaniasis in northeastern Brazil using remote sensing technology for environmental categorization of rain and region influences on leishmaniasis. Am J Trop Med Hyg. 2002;67:648–655. doi: 10.4269/ajtmh.2002.67.648. [DOI] [PubMed] [Google Scholar]
- 29.Bradley RD, Hanson JD, Amman BR, Baxter BD, Carroll DS, Durish ND, Haynie ML, KageYama M, Longhofer LK, Mendez-Harclerode FM, Reeder SA, Suchecki JR, Ruthven DC, Cajimat MN, Milazzo C, Milazzo ML, Fulhorst CF. Rapid recovery of rodent populations following severe drought. Southwest Nat. 2006;51:87–93. [Google Scholar]
- 30.Gonzalez C, Wang O, Strutz SE, Gonzalez-Salazar C, Sanchez-Cordero V, Sarkar S. Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4:e585. doi: 10.1371/journal.pntd.0000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali A, Nayar JK. Invasion, spread, and vector potential of Aedes albopictus in the USA and its control possibilities. Med Entomol Zool. 1997;48:1–9. [Google Scholar]
- 32.McHugh CP. Distributional records for some North American sand flies, Lutzomyia (Diptera: Psychodidae) Entomol News. 1991;102:192–194. [Google Scholar]
- 33.Mead DG, Cupp EW. Occurrence of Lutzomyia anthophora (Diptera: Psychodidae) in Arizona. J Med Entomol. 1995;32:747–748. doi: 10.1093/jmedent/32.5.747. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention Practical Guide for Laboratory Diagnosis of Leishmaniasis. 2011. http://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis.pdf Available at. Accessed September 12, 2011.
- 35.Centers for Disease Control and Prevention Resources for Health Professionals. 2012. http://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html Available at. Accessed October 25, 2012.
- 36.World Health Organization, 2010 . Control of the Leishmaniasis: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases. Geneva: Geneva: World Health Organization; March 22–26, 2010. [Google Scholar]