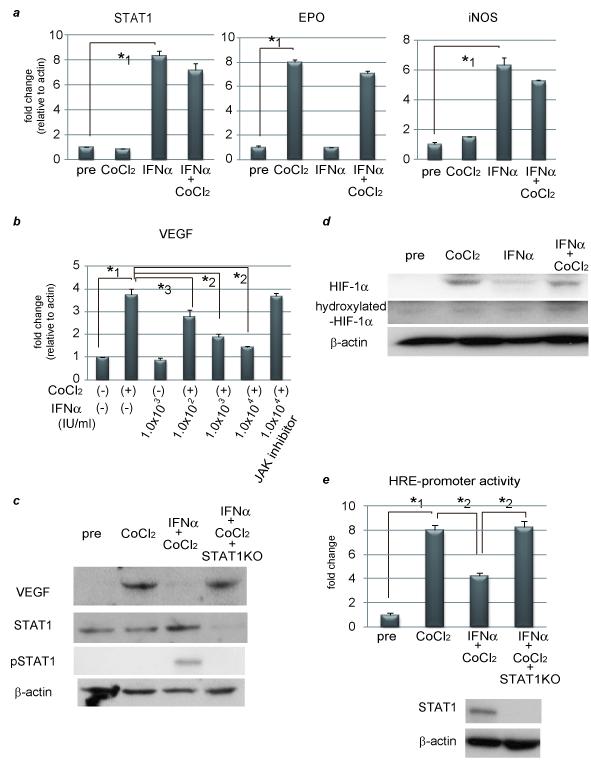
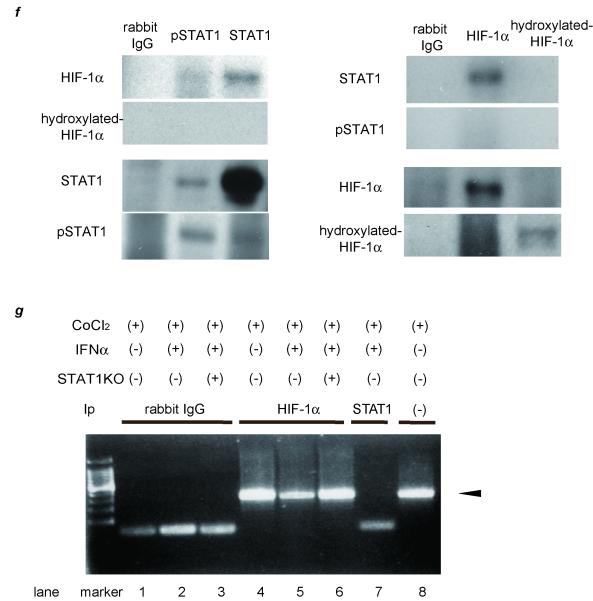
Figure 1. IFNα suppresses VEGF expression through inhibiting HRE-promoter activity by interaction between STAT1 and HIF-1 complex.
(a) (b) Expression levels as detected by real-time RT-PCR analysis. Cells were treated with 100 μM CoCl2 and/or 1.0 × 103 IU/ml (a) or various concentrations of IFNα (b) for 24 hours. To block JAK-STAT signal transduction, 100 nM of JAK inhibitor 1 was pretreated 1 hour before stimulation. (c) Activation of STAT1 and expression of STAT1 and VEGF as detected by western blot analysis. PLC/PRF/5 cells were transfected with control siRNA or siRNA for STAT1 and subjected to each stimulation for 24 hours. (d) Expression levels of HIF-1α and hydroxylated-HIF-1α as detected by western blot analysis. (e) Cells were cotransfected of the pGL2TkHRE plasmid or the pGL2Tk plasmid (as a control vector) with the pRLtK plasmid (Promega). The cells were then stimulated with 100 mM of CoCl2 units/ml and/or 1.0 × 103 IU/ml of IFNα, or left unstimulated, and subjected to dual luciferase assay. The relative light unit of the unstimulated sample was considered as 1 and the data were expressed as mean ± S.D. Lower panel shows expression levels of STAT1 as detected by western blot analysis. (f) Cellular lysates from CoCl2 and IFNγ-treated PLC/PRF/5 cells were immunoprecipitated with nonspecific γ-globulin (lane 1) and antibodies against pSTAT1 (left panel, lane 2), STAT1 (left panel, lane 3), HIF-1α (right panel, lane 2), and hydroxylated-HIF-1α (right panel, lane 3), and the immunoprecipitates were subjected to western blot analysis to detect each protein. (g) ChIP assay with HIF1α or STAT1 antibody. Cells were transfected with siRNA for STAT1 or control, and stimulated with CoCl2 for 24 hours and/or IFNα for 3 hours. The immunoprecipitated DNA was purified and the region from −1386 to −1036 base pairs of the human VEGF promoter was amplified by PCR (35 cycles). Cell lysates without Ip was used as a positive control (lane 8). Asterisks indicate significant differences (*1, P<.001, *2, P<.01, *3, P<.05).