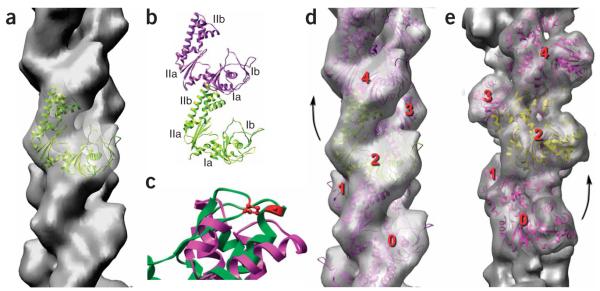
Figure 3.
Filaments of ParM have the opposite handedness to that of F-actin. (a–d) The cryo-EM reconstruction of ParM is shown as a transparent gray surface in a and d, and a model for the ParM subunit (ribbon representation) has been fit into this volume. The four subdomains of the ParM subunit are labeled in b; the strongest interface in the ParM filament is between subdomain IIa of one subunit and subdomain Ia of a subunit above it. c shows a close-up view of an alignment of ParM subdomain IIa (magenta) with actin subdomain 4 (green, with actin residues 204 and 247 in red). Actin residues 204 and 247 are expected to be part of the subunit-subunit interface in F-actin, as mutation of these residues results in loss of polymerization35. The overall structural conservation between actin and ParM does not extend to this region, consistent with different filamentous interfaces. (e) For comparison, a reconstruction of F-actin is shown. In F-actin (e), subunit 2 is rotated from subunit 0 by a right-handed rotation that is ~28° on average. In ParM (d), subunit 4 is rotated from subunit 2 by a left-handed rotation that is ~29° on average.