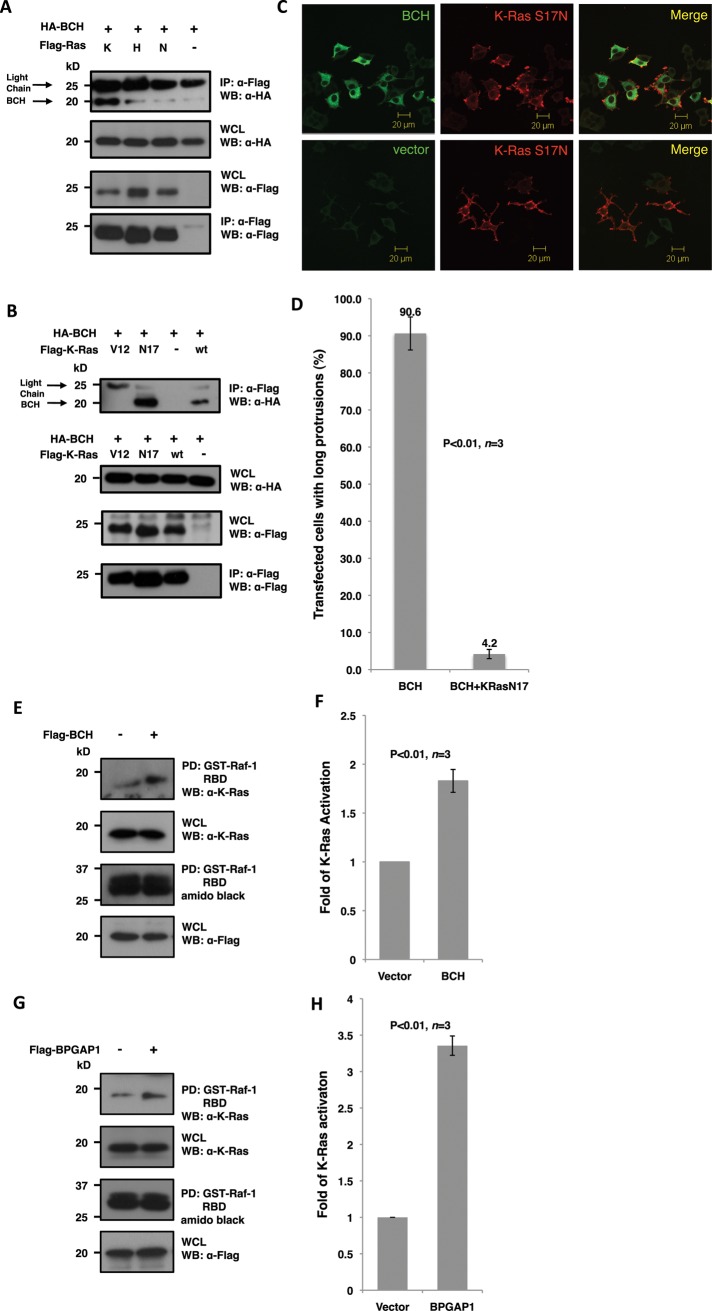
FIGURE 3:
BCH domain of BPGAP1 targets K-Ras and promotes its activation. (A and B) Lysates from HEK293T cells expressing relevant FLAG- and HA-tagged constructs were immunoprecipitated (IP) with anti-FLAG beads and analyzed by SDS–PAGE and Western blotting. Appropriate antibodies were used to detect bound proteins (top panel) and loading control (bottom panel) and for verification of protein expression (middle panels). BCH domain interaction with Ras isoforms K-, H-, and N-Ras shows a strong interaction with the K-Ras isoform (A) with a preference to the dominant-negative S17N mutant (B). (C) PC12 cells overexpressing Flag-BCH and HA-K-Ras-S17N were made quiescent followed by 48 h of EGF (100 ng/ml) stimulation before they were processed by indirect immunofluorescence for confocal microscopy. K-Ras-S17N blocks bipolar neurites caused by the BCH domain. Scale bars, 20 μm. (D) Quantitative representation of the data depicted in (C) as mean of n = 3, p < 0.01, error bars represent SEM. (E and G) BCH domain (E) and full-length BPGAP1 (G) increase K-Ras activation. HEK293T cells expressing Flag-BCH (E), Flag-BPGAP1 (G), or vector control were made quiescent before stimulating with EGF (100 ng/ml) for 5 min. Lysates were incubated with Raf-1–RBD immobilized on beads for the K-Ras activation assay and subsequent SDS–PAGE and Western analysis as described in Materials and Methods. Bound active GTPases (top panel) and endogenous K-Ras protein expression were detected using K-Ras antibodies. Equal loading of GST fusion is observed by Amido Black staining. Experiments were performed in triplicate, and mean band intensities relative to control are plotted in (F) and (H) ±SEM, n = 3; p < 0.01.