Abstract
Engineered nanomaterials, defined as having at least one dimension smaller than 100 nm, have revolutionized many technology sectors ranging from therapeutics and diagnostics to environmental monitoring and remediation. This has resulted in a rapid increase in their manufacture over the past few years, accompanied by an increased human exposure potential. However, understanding of the interactions of nanomaterials with biological systems is still rudimentary. We have described that an environmentally and medically relevant nano metal (cerium dioxide) can affect primary human monocyte viability and interact with programmed cell death pathways leading to apoptosis and autophagic cell death. Cerium dioxide nanoparticles (CeO2 NPs)-induced autophagy acts as a prodeath mechanism and leads to increased cytotoxicity of human monocytes. A better understanding of the implication and biological significance of CeO2 NPs-induced autophagy and apoptosis will help us understand the risks associated with its uses and develop safer nanomedicine.
Keywords: autophagy, apoptosis, nanoparticle, cerium dioxide, human monocyte
With the tremendous increase of engineered nanomaterial use in various sectors of everyday life, the potential for deleterious effects of nanomaterials has become a major concern. In the case of humans, ecosystem and environmental injury could result from exposure. The potential of adverse health effects is expected to further increase in the near future, as a significant number of newly developed nanomaterials is being tested for their potential application in nanomedicine. Among these materials, cerium dioxide nanoparticles have a potential for environmental and human exposure because of significant industrial, environmental, and proposed pharmaceutical applications. A very significant environmental application of CeO2 NPs is as a diesel fuel additive to increase fuel efficiency and reduce particulate emissions. A Health Effects Institute report calculated that such addition of diesel-additive nano CeO2 increased CeO2 NPs emissions up to 22 million pounds annually in the European Union by the year 2010. For these reasons, the Organization for Economic Cooperation and Development has included CeO2 NPs in the priority list of the nanomaterials requiring urgent evaluation.
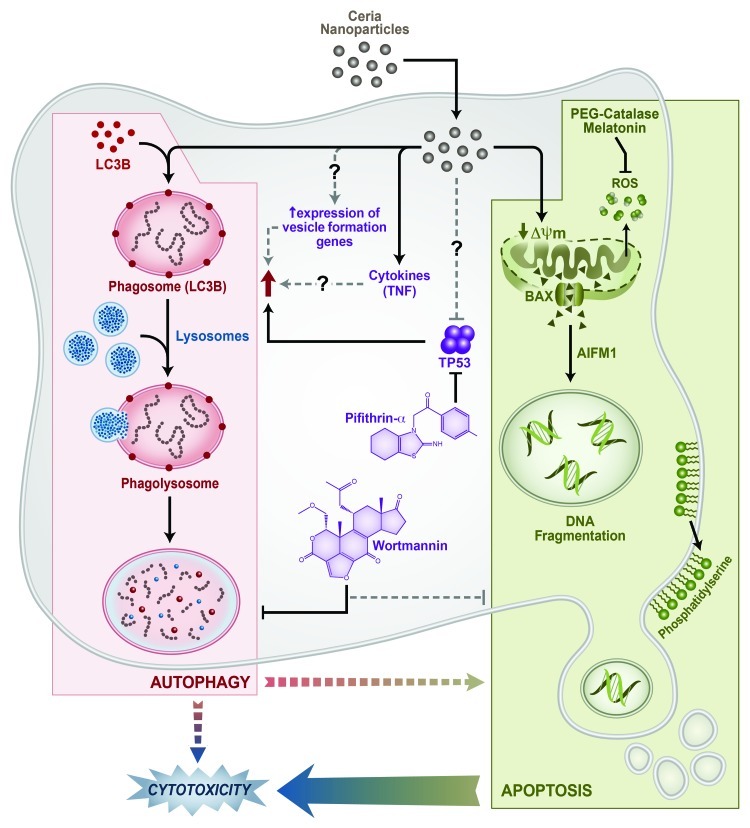
We studied the effect of CeO2 NPs in primary human monocytes. CeO2 NPs induce a significant decrease of cell viability in a time- and dose-dependent manner through the induction of caspase-independent apoptosis involving apoptosis-inducing factor, mitochondrion-associated, 1(AIFM1) induction and mitochondrial damage (Fig. 1). In parallel, a significant increase in autophagy is observed after exposure to CeO2 NPs. Interestingly, the increase in autophagy acts as a prodeath mechanism rather than a prosurvival mechanism, as autophagy inhibition by wortmannin leads to a decrease in apoptotic cells and an increase in viability. Recent data suggest that tumor suppressor protein TP53 can act as an important part of both apoptosis and autophagy processes by either inducing or inhibiting them. In particular, a localization-dependent (cytoplasmic vs. nuclear) control of TP53 over the autophagy process has been shown. We found that TP53 has no significant effect on the apoptosis induced by CeO2 NPs in human monocytes; however, it controls the autophagic events. Pharmacological inhibition of TP53 (by pifithrin-α) significantly increases the number of cells containing autophagic vesicles. On the one hand, this observation confirms the role of TP53 in the autophagy induction, and on the other hand indicates the possibilities of direct effects of CeO2 NPs on cellular/cytoplasmic TP53. We hypothesize that CeO2 NPs have effects on cytoplasmic TP53, as we do not observe CeO2 NPs inside the nucleus of monocytes. Indeed, various literature reports describe observed effects of NPs as a consequence of their interaction with proteins, leading to changes in protein conformation and irreversible loss of activity (in the case of enzymes). Further experimentation is warranted to understand this phenomenon in the case of CeO2 NP exposure.
Figure 1. Pathways of CeO2 NPs-induced cell death in primary human monocytes. CeO2 NPs induce apoptosis involving mitochondrial damage (loss of membrane potential, activation and mitochondrial relocation of BAX) and apoptosis-inducing factor, mitochondrion-associated, 1 (AIFM1) induction. Modulation of autophagic events (increased LC3B-stained autophagic vesicles, monodansylcadaverine-stained autophagosomes/autolysososmes and increased lysosomal contents) decease the cytotoxicity and apoptosis of monocytes. Pharmacological inhibition of TP53 results in an increase in autophagic events, while apoptosis remains unchanged.
An understanding of the molecular mechanisms of effects of nanomaterials will be critical to future studies of potential risks of exposure to these new materials. Among the various mechanisms of toxicity of nanomaterials, autophagy has recently emerged as one of the leading pathways. A wide number of nanomaterials has been shown to perturb autophagy including fullerene/fullerenol, silica, gold, iron oxide, rare earth metal oxides, titanium dioxide and quantum dots. Despite the fact that the materials mentioned above were shown to either induce autophagy or block the autophagic flux, very little is known about the mechanisms behind these events. In particular, very little is known as to how different NPs trigger or block autophagic events. Proposed mechanisms include overexpression of genes involved in vesicle formation and NPs acting as foreign endosomal pathogens. A more probable explanation could be the direct interaction of CeO2 NPs with endosomal/lysosomal compartments, since NPs have been seen to accumulate in these compartments. Various cytokines including tumor necrosis factor (TNF/TNFα) can induce autophagic events. Indeed, we observed a significant increase in TNF after exposure to CeO2 NPs. However, the role of this increase in TNF in the induction of autophagy needs to be established.
The observed results may have significant implications in terms of human health. Monocytes work as integral components of the immune response by initiating or amplifying inflammatory pathways and act as a link between innate and adaptive immune components. Increased cytotoxicity of monocytes can result in impaired early immune response to foreign agents/infection. Moreover, monocytes are precursors of important antigen-presenting cells (macrophages and dendritic cells). Recently, it has been shown that autophagy in antigen-presenting cells can lead to the initiation and/or progression of autoimmune processes.
Further in vivo studies are currently ongoing in our laboratory to establish the biological relevance of CeO2 NP-induced monocyte apoptosis and autophagy. Existing literature suggests that NP-induced autophagic events have important in vivo implications, for example, autophagy induction by polyamidoamine NPs causes acute lung injury in mice. Moreover, others have postulated that autophagy can act as a potential link between nanoparticulate pollution and risk of neurodegenerative disorders. However, in therapeutics autophagy induction improves the efficacy of cancer chemotherapy and vaccination. Various applications of nanomaterials are under development based on their potential to interact with autophagy pathways. Exploitation of the potential of NPs to modulate autophagy may thereby be a beneficial avenue in certain therapeutic uses. However, concerns over the potential deleterious consequences of autophagy will similarly require much additional research.
Acknowledgments
This work was supported (in part) by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (NIEHS).
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/22266